All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Current and future use of BCMA-directed CAR T-cell therapy in MM
Do you know... How did overall response rates (ORR) to ide-cel compare between data obtained from the KarMMa clinical trial and a real-world analysis?
Chimeric antigen receptor (CAR) T cells are a relatively recent development in multiple myeloma (MM), with the first U.S. Food and Drug Administration (FDA) approvals in 2021. The integration of these novel therapies has led to significant changes in the MM treatment landscape, with unprecedented response rates.1 The potential demonstrated in clinical trials has prompted further research into the expansion of CAR T-cell therapies into additional indications.1
The Multiple Myeloma Hub is pleased to provide a summary of the current and future applications for B-cell maturation antigen (BCMA)-directed CAR T-cell therapies in the treatment of MM.
Chimeric antigen receptor
CARs are a type of fusion protein designed to target specific cell surface antigens by genetically modifying a patient’s own T cells, giving them the ability to recognize and bind to specific tumor antigens (Figure 1).1
Figure 1. Overview of the CAR T-cell manufacturing process*
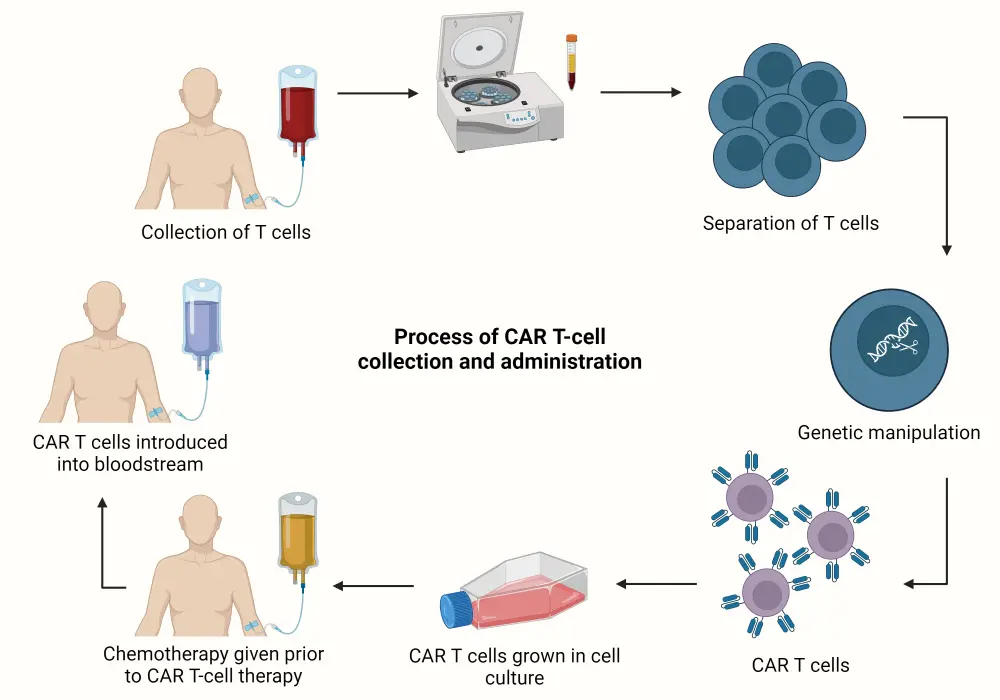
CAR, chimeric antigen receptor.
Created with BioRender.com.
*Adapted from Sheykhhasan, et al.1
Current use of CAR T-cell therapy in MM
Two CAR T-cell therapies are currently approved in MM: idecabtagene vicleucel (ide-cel) and ciltacabtagene autoleucel (cilta-cel). Both agents were granted FDA accelerated approval for the treatment of relapsed/refractory MM (RRMM) with four or more prior lines of therapy including a proteasome inhibitor (PI), immunomodulatory drug (IMiD), and anti-CD38 antibody.2
On April 5, 2024, the FDA granted approval to an extended indication of ide-cel for the treatment of triple-class-exposed RRMM after two or more prior therapies, including an IMiD, PI, and anti-CD38 antibody. On April 22, 2024, The European Commission went on to approve cilta-cel in RRMM after one or more prior therapies – including an IMiD or PI – in those with lenalidomide-refractory disease and disease progression on the last therapy.
BCMA
Both approved CAR T-cell products are engineered to target BCMA, which is one of the most commonly targeted surface proteins in MM due to its highly selective expression on mature B lymphocytes and plasma cells. BCMA signaling is also heavily involved in MM disease activity and tends to increase expression as the disease progresses. Furthermore, BCMA has an important role in the differentiation of neoplastic plasma cells due to interaction with proliferation-inducing ligand (APRIL) and B-cell activating factor (BAFF), making BCMA an optimal target for immunotherapies.2
Real-world safety and efficacy data for ide-cel3
Ide-cel for the treatment of RRMM was initially approved based on data from KarMMa (NCT03361748). However, a majority of real-world patients are heavily pre-treated or have comorbidities that would have made them ineligible for the pivotal clinical trial. Real-world data for standard-of-care ide-cel in 821 patients were presented at the American Society of Hematology (ASH) Annual Meeting and Exposition 2023. The data demonstrated a largely comparable safety and efficacy profile between the real-world and trial cohorts.
- Response rates were similar between real-world patients and those from KarMMa (Figure 2).
- Progression-free survival (PFS) rates were comparable between real-world patients at 9 months and 8.8 months in KarMMa.
- Incidence of immune effector cell-associated neurotoxicity syndrome (ICANS) was observed to be higher in the real-world cohort; however, Grade ≥3 ICANS rates were similar at 5% and 3%, respectively.
Figure 2. Response data from real-world and KarMMa cohorts*
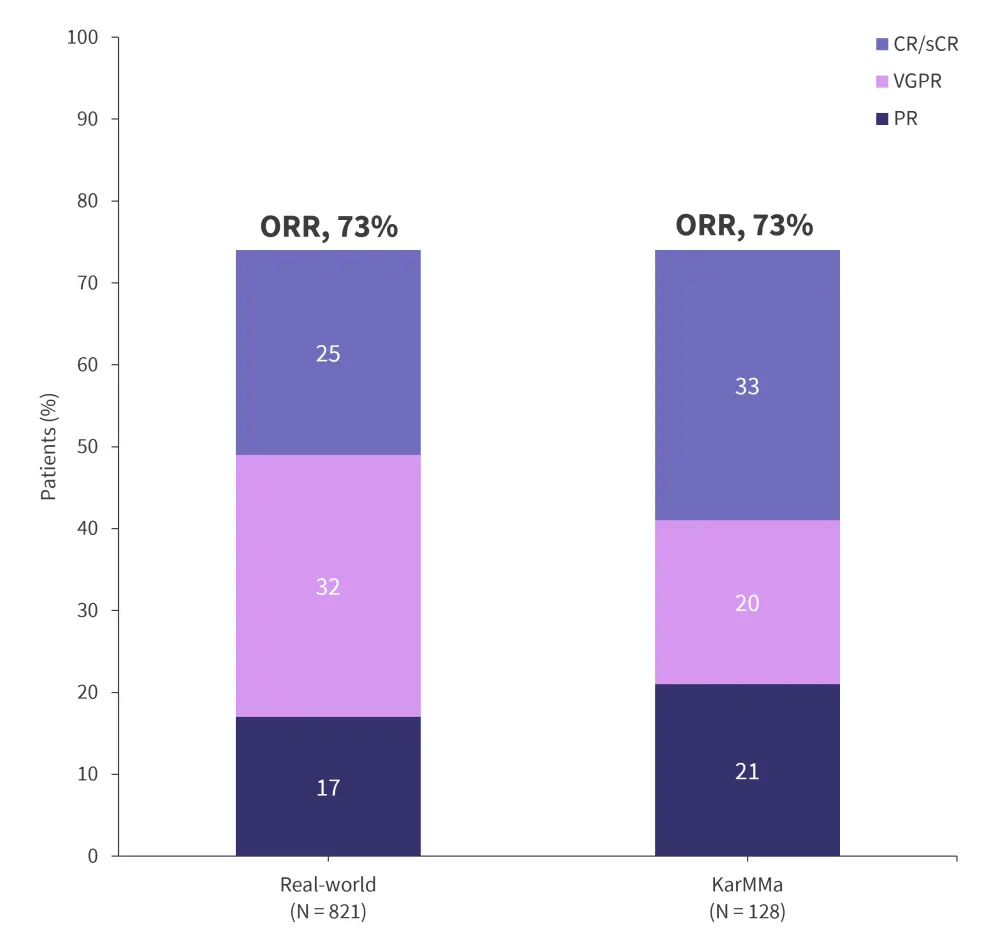
CR, complete response; ORR, overall response rate; PR, partial response; sCR, stringent complete response; VGPR, very good partial response.
*Data from Sidana.3
Challenges
While CAR T-cell therapies have resulted in notably high response rates, multiple barriers remain to real-world treatment access. Access to CAR T-cell therapy in general is limited, with time consuming production and many regions, including Central and South America, having no access to myeloma-specific CAR-T products.
A review of patients referred for CAR T-cell therapy recorded 40% going on to receive treatment, with approximately 25% dying while waiting for therapy.2 CAR T-cell therapies are often also associated with high rates of toxicities, including cytokine release syndrome (CRS) and neurotoxicity. To mitigate the risks of neurotoxicity and CRS, drugs including the humanized anti-IL6 receptor antibody tocilizumab and siltuximab, as well as the IL-1 receptor antagonist anakinra, were approved by the FDA.4
Future use of CAR T-cell therapy in MM
CAR T cells, as well as T-cell engaging therapies in general, are being increasingly investigated in earlier lines of therapy for the treatment of MM. The rationale for use in earlier lines has been previously reported by the Multiple Myeloma Hub here, and includes key points such as:5
- Increased fitness of T cells
- Increased immunogenicity of tumor cells
- Improved tolerability observed in clinical trials
- Lower likelihood of pretreatment with agents that have a negative impact on CAR T-cell efficacy
Most notably, it is argued that treatments with the highest efficacy rates should be implemented as early as possible in the treatment pathway when patients are more likely to be fitter and, therefore, may get the greatest benefit from the therapy.
In addition to investigation for use in earlier lines, ide-cel is also under investigation in other indications such as high-risk disease (KarMMa-2), in combination with other therapies (KarMMa-7), and as a maintenance therapy alongside lenalidomide (KarMMa-9).
KarMMa-26
- KarMMa-2 (NCT03601078) is an open-label, multicohort, phase II clinical trial evaluating the safety and efficacy of ide-cel in patients with RRMM and high-risk disease features (Figure 3).
Figure 3. KarMMa-2 cohorts*
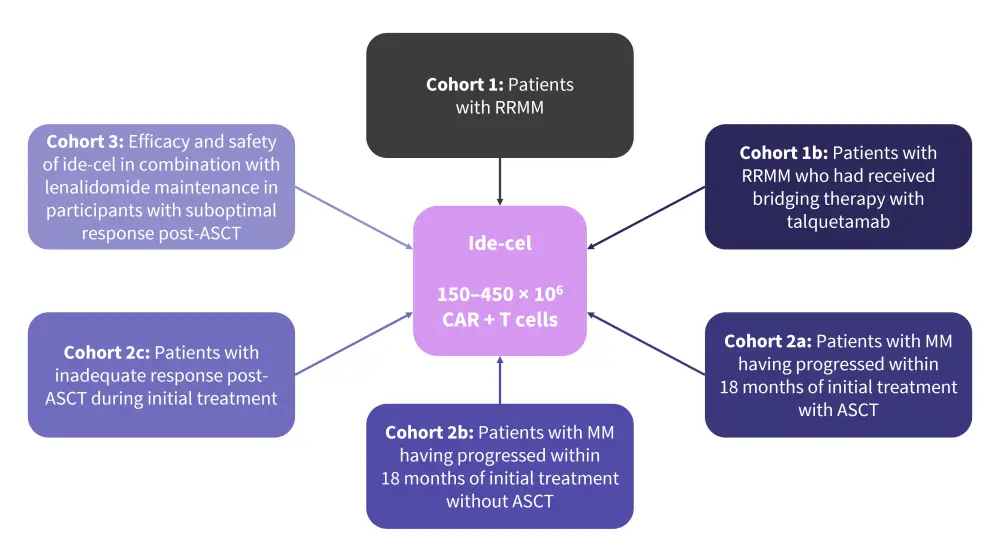
ASCT, autologous stem cell transplant; CAR, chimeric antigen receptor; MM, multiple myeloma; RRMM, relapsed/refractory multiple myeloma.
*Data from ClinicalTrials.gov6
KarMMa-77
- KarMMa-7 (NCT04855136) is an open-label, phase I/II, dose-finding and dose-expansion clinical trial evaluating combination therapies with ide-cel for the treatment of RRMM.
- Adult patients with RRMM who had received either 1–3 or ≥3 prior regimens were eligible.
- Patients are to be allocated to three non-randomized treatment arms (Figure 4).
Figure 4. KarMMa-7 study design*
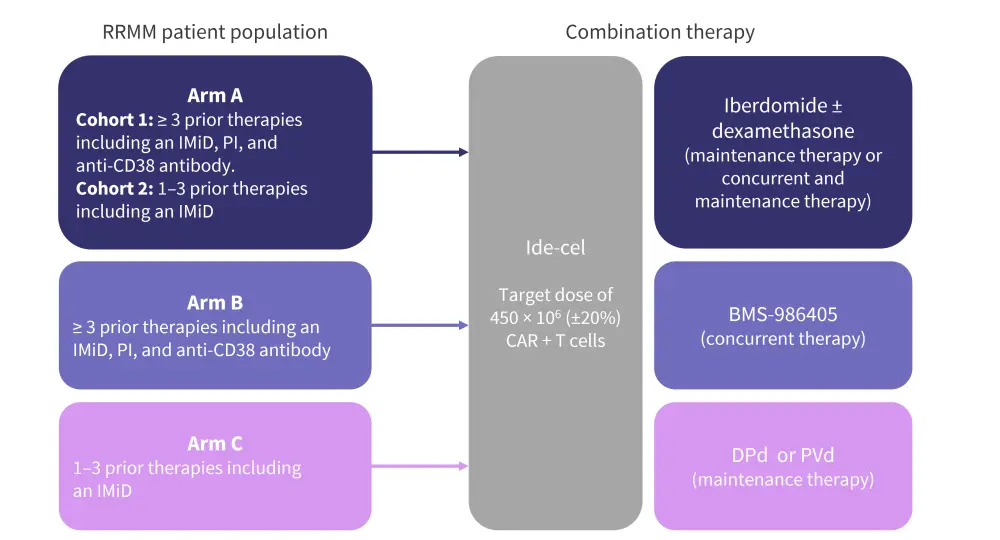
CAR, chimeric antigen receptor; DPd, daratumumab-pomalidomide-dexamethasone; IMiD, immunomodulatory agent; PI, proteosome inhibitor; PVd, pomalidomide-bortezomib-dexamethasone.
*Adapted from Raje, et al.7
KarMMa-98
- KarMMa-9 (NCT06045806) is an ongoing phase III trial comparing the efficacy and safety of ide-cel plus lenalidomide as a maintenance therapy vs lenalidomide alone in patients with newly diagnosed multiple myeloma (NDMM) who had a suboptimal response to stem cell transplant (Figure 5).
- Patients will be randomly assigned to receive ide-cel plus lenalidomide or standard-of-care lenalidomide maintenance.
- Randomization will be stratified, based on the stage of disease, induction therapy, and the degree of treatment response post-ASCT.
- Combining ide-cel with standard-of-care maintenance therapy is expected to improve clinical outcomes and extend PFS in patients with clinically high-risk NDMM.
- A total of 618 eligible patients with NDMM have enrolled so far, with recruitment ongoing.
Figure 5. KarMMa-9 study design*
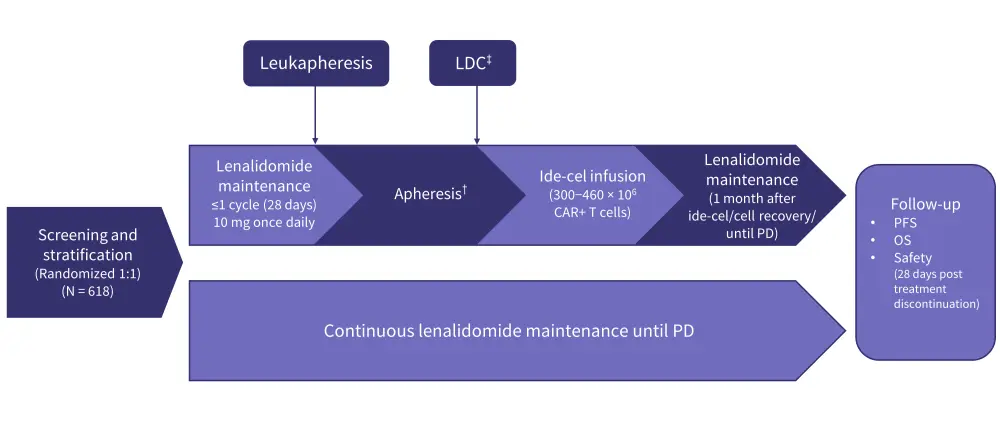
CAR, chimeric antigen receptor; ide-cel, idecabtagene vicleucel; LDC, lymphodepleting chemotherapy; OS, overall survival; PD, progressive disease; PFS, progression-free survival. *Adapted from Mateos, et al.8
†Apheresis to be performed within 14−42 days after last dose of R.
‡Fludarabine 30 mg/m2 and cyclophosphamide 300 mg/m2 on Days -5, -4, and -3 prior to ide-cel infusion.
Conclusion
CAR T-cell therapies have resulted in previously unseen response rates in MM. However, they are accompanied by a multitude of challenges in development, administration, and toxicities. Currently, CAR T-cell therapies are typically used in later lines of therapy, in the RR setting. Nevertheless, the recommendation for use of CAR T-cell therapies in earlier lines of treatment is a recurring theme amongst experts, with the potential to lower disease burden, increase response rates, and improve ability to tolerate toxicities. Multiple clinical trials into the expanded use of CAR T-cell therapies are ongoing, with pivotal data anticipated in the coming years and potential for significant changes in the MM treatment landscape.
This educational resource is independently supported by BMS. All content is developed by SES in collaboration with an expert steering committee; funders are allowed no influence on the content of this resource.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?


