All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Continuous versus fixed duration therapy in multiple myeloma: The great debate
Featured:
Considerable advances have been made in the treatment of multiple myeloma (MM) over the past 2 decades, including alternatives to alkylator-based induction regimens and the addition of novel agents such as daratumumab, elotuzumab, ixazomib, pomalidomide, and panobinostat, among others.1,2 These new agents have instigated changes in diagnostic criteria, staging, and response criteria, including the addition of definitions for minimal residual disease (MRD) negativity.2 The treatment of newly diagnosed MM (NDMM) is currently driven by transplant eligibility and risk stratification and includes continuous lenalidomide (with or without bortezomib) maintenance (Figure 1), but is the maintenance paradigm shifting?
Figure 1. Current treatment algorithms for NDMM in transplant eligible and transplant ineligible patients*
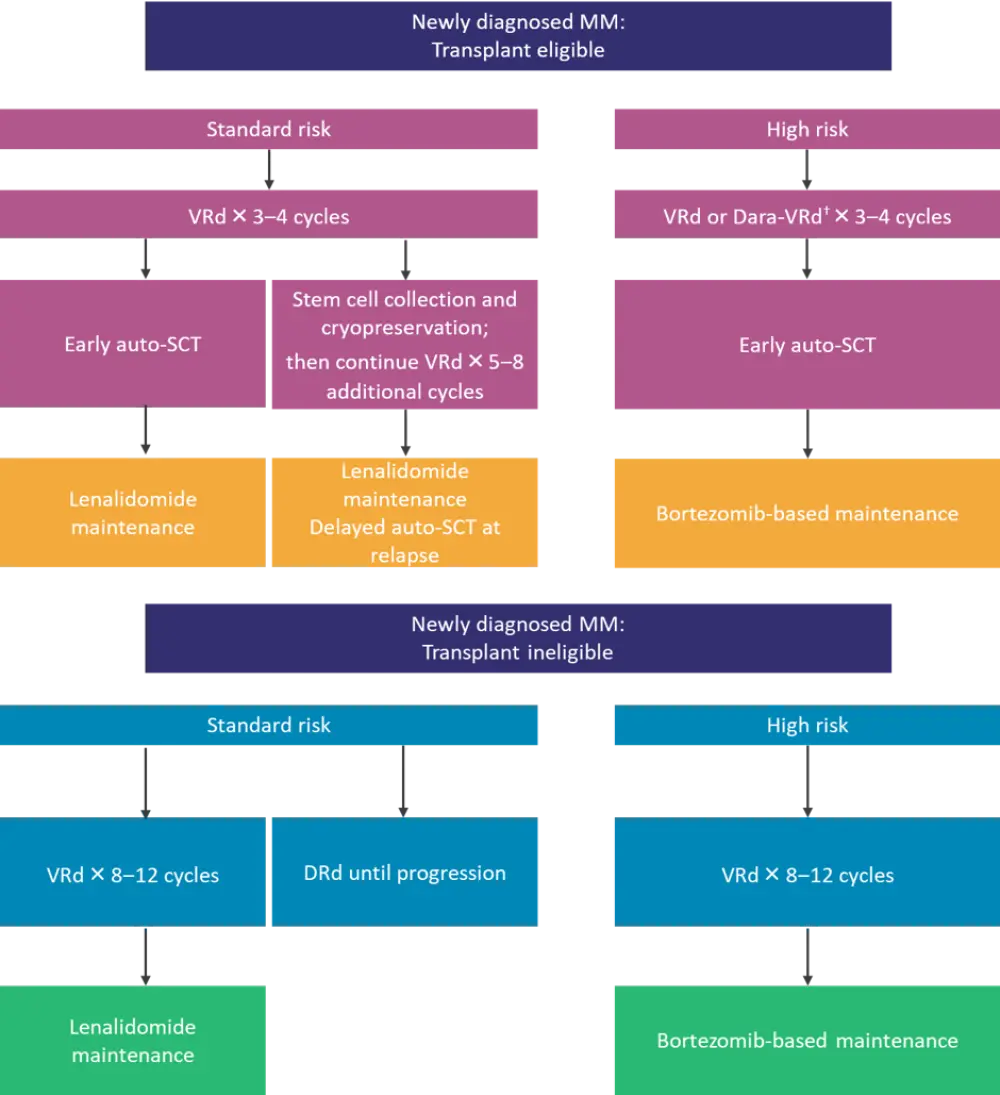
auto-SCT, autologous stem cell transplant ; Dara-VRd, daratumumab, bortezomib, lenalidomide, dexamethasone; DRd, daratumumab, lenalidomide, dexamethasone; MM, multiple myeloma; NDMM, newly diagnosed multiple myeloma; VRd, bortezomib, lenalidomide, dexamethasone.
*Adapted from Rajkumar et al.2
†Dara-VRd is recommended for high-risk patients, particularly those with double- or triple-hit MM.
At the 18th International Myeloma Workshop (IMW) in September 2021, Luciano Costa, from the University of Alabama at Birmingham, Birmingham, US, and Philip McCarthy, from the Roswell Park Comprehensive Cancer Center, Buffalo, US, debated whether patients with NDMM can be given a treatment-free interval, or a continuous therapy is still the standard of care.3
Luciano Costa: We can’t call it cure until we can stop therapy
While the current paradigm for upfront therapy in patients with MM is continuous therapy with lenalidomide (adding bortezomib in high-risk patients) due to its superiority regarding progression-free survival (PFS) and overall survival (OS), there are some caveats3,4:
- Intent vs reality: Real-world data from the US, where lenalidomide is the advised standard therapy, shows that the median duration of lenalidomide maintenance is as little as 21 months. This means that the intent—continuous therapy until disease progression—ends up being a limited duration therapy in reality.
- The continuous approach assumes that this treatment is optimal for all patients, regardless of fitness, disease biology, or depth of response.
- The evidence for continuous therapy with lenalidomide was defined in a setting of less active therapy; with induction and consolidation regimens inferior to current treatment options, and as upfront therapy continues to improve, the idea of continuous maintenance should be revisited.
- There is a two- to three-fold increase in the risk of second primary malignancies (SPM) with lenalidomide.
- The cost and inconvenience of continuous therapy is considerable.
Updated results from the 8-year follow up of the IFM 2009 study, in which patients received only 1 year of fixed-duration lenalidomide, showed that among patients who achieve MRD negativity, the majority are still progression-free 7 years after interruption of treatment.5 Costa argues that deferring lenalidomide for 7 years means avoiding the approximately three-fold increase in risk of SPM, as well as dozens of laboratory tests and doctor visits, the diarrhea, fatigue, and rash commonly associated with lenalidomide, and the cost associated with that prolonged therapy.3
Costa3 states that, while lenalidomide maintenance until disease progression does work and is the right choice for some patients, it is not the right choice for all patients. Results from Part 2 of the CASSIOPEIA trial, which compared daratumumab maintenance vs observation, showed that three out of four patients were without disease progression 3.5 years after the end of consolidation.6 Early results of the MASTER trial—which is investigating daratumumab, carfilzomib, lenalidomide, and dexamethasone (dara-KRd), autologous transplantation, and MRD response-adapted treatment duration and cessation in NDMM—demonstrate that confirmed MRD negativity is achievable in about 80% of patients with response-adapted therapy, even across cytogenetic risk categories.7
What is the proposed alternative for continuous maintenance therapy for all patients, and how can we identify which patients will benefit from an alternative approach? Costa suggests treatment-free observation with MRD surveillance, noting that the risk of omitting maintenance must be weighed against quality of life, cost, and toxicity associated with continuous therapy.3
Philip McCarthy: We still do not know how to best individualize therapy
On the other side of the debate, McCarthy says that we cannot give patients treatment-free intervals until we are able to identify patients who can stop therapy without negative impact. He maintains that most patients with MM require continuous therapy to maintain disease control, and while some patients may benefit from treatment-free observation, there are currently no reliable biomarkers to individualize treatment, and there are no approved surrogates (such as MRD or immune profiling) for PFS and OS.3
McCarthy notes that, regarding maintenance treatment and median PFS, continuous lenalidomide is associated with longer PFS compared with fixed duration lenalidomide or lenalidomide that is stopped early. He also considers the MRD analysis from the IFM 2009 trial, noting that progression occurred even in MRD-negative patients and that most patients in the trial were MRD positive.3,5
Results from the Myeloma XI and EMN02/HO95 trials showed that continuous maintenance with lenalidomide was associated with a significantly longer median PFS compared with observation, and while MRD-negative patients do well under observation, some of them still do progress. For patients who are MRD positive and become negative, a benefit is also seen; however, for patients who are MRD negative and turn MRD positive (or are MRD positive and stay positive), the PFS with observation is inferior.8,9
In relapsed/refractory (R/R) patients, data are also in support of continuous maintenance therapy:
- In the CASTOR trial, comparing 6 months of bortezomib-dexamethasone with or without continuous daratumumab, PFS was superior in the daratumumab arm (16.7 months vs 7.1 months), although the OS was similar between the two arms (59% vs 52%).10
- MRD negativity was superior in the daratumumab arm, but some of those patients still had disease progression, and the vast majority of the patients had MRD-positive disease.
- In the POLLUX trial, comparing lenalidomide-dexamethasone with or without daratumumab, PFS was 44.5 months in the daratumumab arm vs 17.5 months in the group that received only lenalidomide-dexamethasone, and the OS at 42 months was 65% vs 57%.11
- Time to subsequent therapy was 23 months for the doublet arm vs a median not reached (estimated 59 months) for the triplet arm.
For transplant eligible patients with MM, therefore, McCarthy supports lenalidomide maintenance until disease progression. For transplant eligible and ineligible patients, continuous therapy improves PFS (and OS in selected studies), and continuous therapy extends PFS for patients with R/R MM. After disease progression, there are multiple salvage options which improve PFS, and early progression after fixed duration therapy diminishes quality of life due to earlier salvage treatment. McCarthy also argues that maintenance therapy is cost effective, as it delays time to more intense treatment lines. He concludes by saying that fixed duration therapy has not proven to be superior to continuous therapy, which has contributed to a median PFS of more than 5 years and a median OS of more than 10 years, and we still do not know how to best individualize therapy.3
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?


 Luciano Costa
Luciano Costa Philip L. McCarthy
Philip L. McCarthy