All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Patients with relapsed/refractory multiple myeloma (RRMM) experience significantly poorer outcomes, with few therapies that elicit a significant response. Bispecific antibodies (bsAbs) and chimeric antigen receptor (CAR) T-cell therapies are two novel therapeutic strategies which have been found to be effective in advanced disease.1
During the Society of Hematologic Oncology (SOHO) Annual Meeting 2023, Ajai Chari presented on the considerations when selecting bsAbs and CAR T-cell therapies for the treatment of RRMM. The Multiple Myeloma Hub is pleased to summarize this presentation.
CAR T-cell therapies1
CAR T-cell therapies are produced by collecting T-cells from the patient and re-engineering them to present specific antigens on their surface which target myeloma cells. There are currently two CAR T-cell therapies approved by the Food and Drug Administration (US FDA) for the treatment of RRMM: idecabtagene vicleucel (ide-cel) and ciltacabtagene autoleucel (cilta-cel). The use of these CAR T-cell therapies results in unprecedented high response rates in patients with RRMM.
- Overall response rate (ORR): Ide-cel 81% and cilta-cel 98%
- Median progression-free survival: ide-cel 12.2 months and cilta-cel 34.9 months
CAR T-cell therapies are administered as a single infusion, which has the benefit of a high treatment-free interval for the patient and a lower rate of protein and genomic loss compared with agents used in a continuous schedule, such as bsAbs.
However, increased rates of high-grade cytokine release syndrome and immune effector cell-associated neurotoxicity limits the number of patients who are eligible for CAR T-cells. Furthermore, CAR T-cells have an extended manufacturing time as they must be engineered for each individual. This means that they may not be appropriate for patients with rapidly progressive disease.
Currently, CAR T-cell therapies are only approved in the heavily pretreated population; however, there may be a benefit of moving it to earlier lines such as:
- Less T-cell exhaustion; better T-cell fitness
- Fewer tumor genomic alterations that come with repeated treatments
Bispecific antibodies1
Bispecific antibodies simultaneously bind to T-cells and to tumor cells, which leads to T-cell activation, proliferation, and ultimately, cell death. BsAbs are significantly more easily accessible than CAR T-cell therapies, often available as an off-the-shelf therapy. They are typically administered over the course of a year or longer in patients with MM, as opposed to a single infusion of CAR T-cells.
Compared with CAR T-cell therapies, bsAbs are an effective treatment, but they do have comparatively lower response rates. Overall response rates and median progression-free survival data for CAR T-cell and bsAb therapies are shown in Figure 1 and Figure 2.
Please note, direct comparison between trials cannot be made due to differences in the enrolled population.
Figure 1. Overall response rates for CAR T-cell and bispecific antibody therapies*
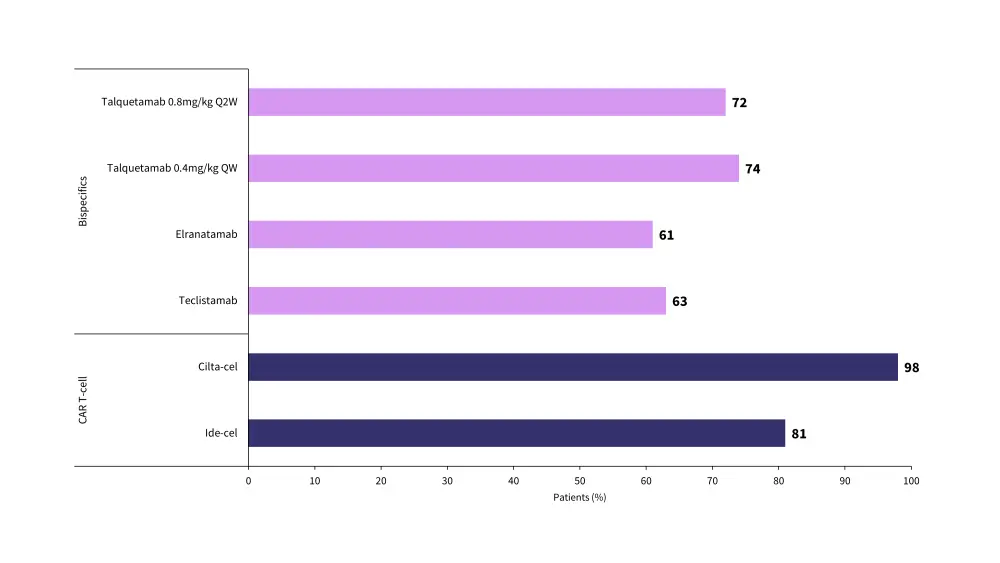
CAR, chimeric antigen receptor; cilta-cel, ciltacabtagene autoleucel; ide-cel, idecabtagene vicleucel; QW, weekly dosing; Q2W, fortnightly dosing.
*Data from Chari.1
Figure 2. Median progression-free survival for CAR T-cell and bispecific antibody therapies*
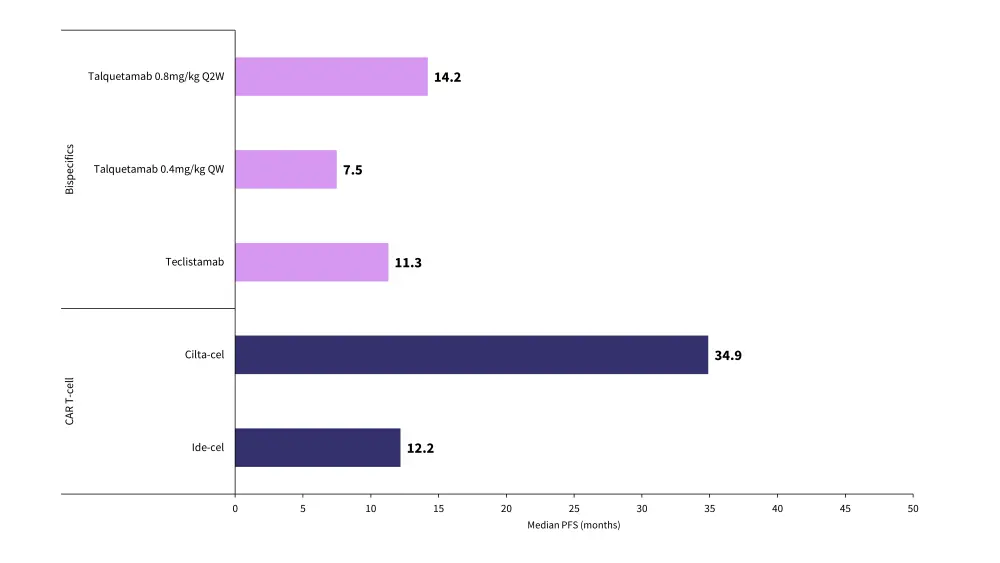
CAR, chimeric antigen receptor; cilta-cel, ciltacabtagene autoleucel; ide-cel, idecabtagene vicleucel; PFS, progression-free survival; QW, weekly dosing; Q2W, fortnightly dosing.
*Data from Chari.1
A further benefit of bsAb is that, due to the repeated dosing, treatments can be modified and even discontinued completely to manage any resulting AEs. This means that bsAbs may be appropriate for a higher proportion of patients with comorbidities who may otherwise not be able to tolerate standard dosing or single-infusion CAR-T.
Conclusion
Both CAR T-cells and bispecific antibody therapies yield high response rates in patients, with CAR T-cells being particularly effective at eliciting a response in the heavily pretreated population.
Overall, while CAR T-cell therapies have been found to result in typically improved outcomes in patients, they are not always appropriate due to extended production time, associated costs, and adverse events. Because of this, CAR T-cell therapies are most appropriate for patients with indolent disease that are able to wait the manufacturing period. Patients with more progressive disease or those unable to tolerate the associated AEs may receive bispecific antibodies. Real-world data following treatment with bsAbs and CAR T-cell therapies may not reflect clinical trial data due to the specific patient populations they are used to treat. Notably, the use of bsAbs primarily in patients with more progressive disease could significantly impact outcomes.
What are the pros and cons of bispecific antibodies and CAR T-cell therapies in myeloma?
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?


 Mohamad Mohty
Mohamad Mohty