All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Johnson & Johnson, Legend Biotech, Pfizer, Roche, and Sanofi. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Carfilzomib, a next-generation proteasome inhibitor, has demonstrated encouraging clinical effects in newly diagnosed and relapsed/refractory multiple myeloma (MM) when combined with other anti-myeloma agents. However, little is known about its efficacy when used as maintenance therapy after autologous stem cell transplantation (ASCT).
Carfilzomib as maintenance for newly diagnosed MM
ASCT followed by lenalidomide maintenance is the current standard of care for patients with newly diagnosed MM who are eligible for transplant. However, some patients may not respond to or tolerate treatment with lenalidomide. Therefore, the high minimal residual disease (MRD) negativity rates achieved with carfilzomib treatment warrant further investigation. The phase II CARDAMON trial (NCT02315716) assessed ongoing carfilzomib treatment versus ASCT and the role of fixed-duration carfilzomib (56 mg/m2 weekly) maintenance following ASCT. During the 18th International Myeloma Workshop, Rakesh Popat provided an update of the CARDAMON trial, and the results have been summarized below.1
Key study results
- The 2-year progression-free survival (PFS) rates with KCd (carfilzomib-cyclophosphamide-dexamethasone) consolidation did not meet the statistical boundary for non-inferiority to ASCT.
- In total, 27.8% of MRD-positive patients converted to MRD-negative post ASCT/consolidation, with more patients converting with ASCT compared with consolidation (39.1% vs 16.1%, respectively; p = 0.004).
- MRD-negative patients at 6 months post maintenance had longer PFS compared with MRD-positive patients (p = 0.004); with higher MRD negativity rates in the ASCT arm compared with the consolidation arm (58.1% vs 40.5%, respectively; p = 0.02).
- MRD-negative patients had superior PFS irrespective of treatment randomization (p = 0.002), with MRD-positive patients after KCd consolidation exhibiting the worst outcomes.
- Carfilzomib maintenance was well tolerated with low withdrawals.
- The most common adverse events (AEs) were hypertension and infections, and there was a higher rate of Grade III−IV AEs and discontinuations/study withdrawals due to AEs during maintenance post ASCT compared with post consolidation (9.1% vs 1.0%, respectively).
For more information, watch our interview with Rakesh Popat below.
After the CARDAMON trial update, what do we know about the role of ASCT and K maintenance in NDMM?
Carfilzomib before and after salvage ASCT
The majority of patients with MM will eventually relapse after ASCT. In this setting, salvage ASCT can be offered to eligible patients as an alternative option with the potential for long-term disease control. However, there are limited data on the optimal induction regimen before salvage ASCT and on the role of maintenance therapy after salvage ASCT.
Gregersen et al. recently performed a randomized study that explored the efficacy and safety of KCd induction before salvage high-dose melphalan with ASCT.2 The study also prospectively investigated carfilzomib and dexamethasone as maintenance therapy after salvage ASCT. We have previously summarized this CARFI trial (NCT02572492) conducted by the Nordic Myeloma Study Group (NMSG); herein, we provide the updated results.
Methods
This was an open-label, phase II trial that included patients with MM from 25 hospitals in Denmark, Finland, Lithuania, Norway, and Sweden.
Eligibility criteria
The study comprised patients with MM with first relapse >1 year after upfront single or double ASCT with ≥2.0 × 106 frozen CD34+ stem cells/kg body weight stored.
Exclusion criteria
Patients were excluded if they had:
- received any MM treatment after the first ASCT, including maintenance therapy, with exceptions of radiotherapy, bisphosphonates, denosumab, and short-term corticosteroids;
- undergone previous treatment with carfilzomib;
- a World Health Organization (WHO) performance status ≥3; or
- significant neuropathy (Grade III–IV, or Grade II with pain).
Treatment schedule
- Patients received four 28-day cycles of KCd.
- Cycle 1 included administration of intravenous (IV) carfilzomib 20 mg/m2 on Days 1 and 2, and IV carfilzomib 36 mg/m2 on Days 8, 9, 15, and 16.
- Cycles 2–4 consisted of IV carfilzomib 36 mg/m2 on Days 1, 2, 8, 9, 15, and 16.
- Oral cyclophosphamide (300 mg/m2 on Days 1, 8, and 15) and oral dexamethasone (20 mg on Days 1, 2, 8, 9, 15, and 16) were administered in each cycle.
- The conditioning regimen comprised IV carfilzomib 27 mg/m2 on Days −2 and −1 and IV melphalan 200 mg/m2 on Day −2.
- Two months following ASCT, patients were randomized (1:1) to either observation or maintenance therapy with IV carfilzomib 27 mg/m2 and oral dexamethasone 20 mg every second week.
- The maintenance dose of carfilzomib was increased to 56 mg/m2 after 4 weeks provided the presence of tolerable side effects.
- The randomization was graded according to relapse 1–2 years or >2 years after ASCT, International Staging System (ISS) stage, and standard-risk versus high-risk cytogenetics.
- Maintenance treatment continued until progression, unacceptable adverse effects, withdrawal of consent, or study termination.
Primary endpoints
There were two primary endpoints; the first was comparison of time to progression (TTP) after upfront ASCT and TTP after salvage ASCT with KCd induction, and the second was comparison of TTP between carfilzomib-dexamethasone maintenance and observation in patients treated with salvage ASCT.
Secondary endpoints
- Toxicity of KCd as induction regimen.
- Toxicity of carfilzomib as part of the high-dose melphalan conditioning.
- Response rates of induction therapy and ASCT.
- Time to bone marrow regeneration (neutrophil and platelet recovery) after ASCT.
- Toxicity of maintenance treatment with carfilzomib-dexamethasone.
- Comparison of overall survival between carfilzomib-dexamethasone maintenance and observation in patients treated with salvage ASCT.
- Patient-reported health-related quality of life.
Results
The study enrolled 200 patients between January 26, 2015, and April 30, 2018; 82 were randomly assigned to the carfilzomib-dexamethasone maintenance arm and 86 to observation after completion of salvage ASCT (Figure 1).
Figure 1. Study profile*
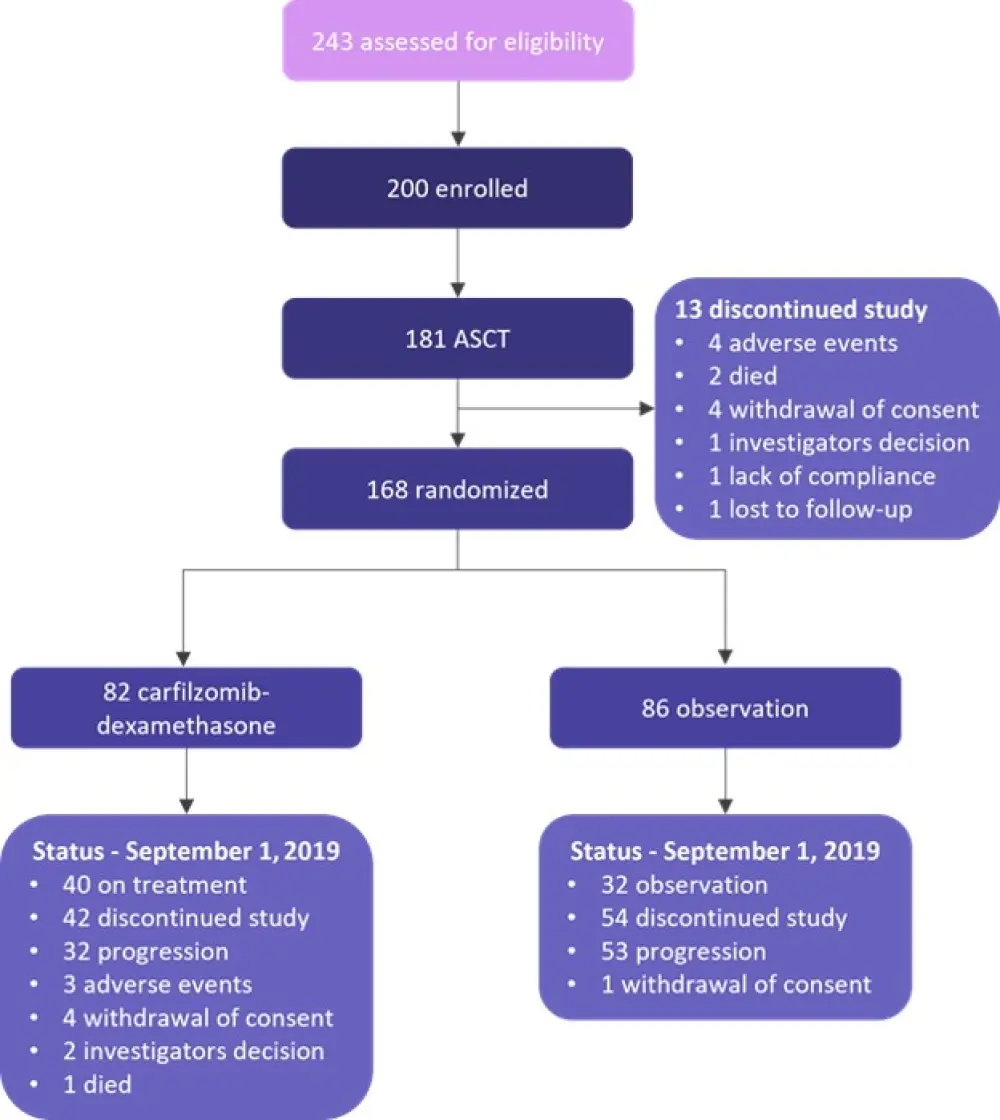
ASCT, autologous stem cell transplantation.
*Adapted from Gregersen, et al.2
Baseline demographic and clinical characteristics at the time of randomization were well-balanced between the two study groups, except for a slightly younger age in the carfilzomib-dexamethasone group versus the control group (p = 0.008) (Table 1). The majority of patients (83.5%) had received previous treatment with bortezomib as part of induction therapy before upfront ASCT. The median follow-up for this analysis was 24.0 months.
Table 1. Baseline demographic and clinical characteristics*
|
BOR-DEX, bortezomib-dexamethasone; CY-BOR-DEX, cyclophosphamide-bortezomib-dexamethasone; CY-DEX; cyclophosphamide-dexamethasone; CY-THAL-DEX, cyclophosphamide-thalidomide-dexamethasone; ISS, International Staging System; KCd, carfilzomib-cyclophosphamide-dexamethasone; MM, multiple myeloma; VAD, vincristine-doxorubicin-dexamethasone. |
|||
|
Characteristic |
All participants treated with KCd |
Carfilzomib group |
Observation group |
|---|---|---|---|
|
Median age, years (range) |
62 (56–66) |
60 (53–64) |
62 (58–67) |
|
Male, % |
58 |
52 |
62 |
|
Time from MM diagnosis, months (range) |
41.3 (30.0–58.4) |
40.4 (30.0–58.5) |
42.0 (30.8–54.7) |
|
Upfront induction regimen, % |
|
|
|
|
VAD |
<1 |
0 |
1 |
|
CY-DEX |
2 |
1 |
1 |
|
CY-BOR-DEX |
73 |
73 |
73 |
|
CY-THAL-DEX |
6 |
7 |
6 |
|
|
11 |
12 |
9 |
|
ISS, % |
|
|
|
|
I |
53 |
57 |
58 |
|
II |
33 |
32 |
28 |
|
III |
9 |
5 |
9 |
|
Missing |
6 |
6 |
5 |
|
Cytogenetics, % |
|
|
|
|
High-risk† |
20 |
17 |
17 |
The median TTP from start of induction therapy was 33.2 months (range, 30.4–37.7 months) for the upfront treatment and 26.7 months (range, 24.2–30.7 months) for the salvage treatment (p < 0.0001). TTP after upfront treatment was an important predictor of TTP after salvage ASCT (p = 0.025): median TTP was 20.7 months (range, 17.8–25.9 months) in patients with remission ≤24 months, and 29.3 months (range, 26.2–35.4 months) in patients with remission ≥24 months. Median TTP after randomization was 25.1 months (range, 22.5 months to not reached) in the carfilzomib-dexamethasone maintenance group and 16.7 months (range, 14.4–21.8 months) in the control group (hazard ratio, 0.46; 95% confidence interval, 0.30–0.71; p = 0.0004). Figure 2 summarizes treatment responses after induction therapy and ASCT.
Figure 2. Response after induction and ASCT*
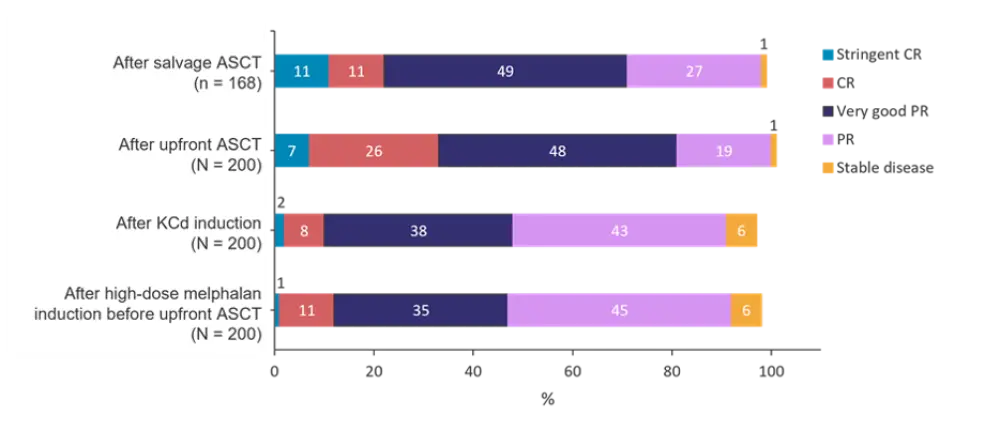
ASCT, autologous stem-cell transplantation; CR, complete remission; KCd, carfilzomib-cyclophosphamide-dexamethasone; PR, partial response.
*Data from Gregersen, et al.2
- There was no significant difference between the quality of treatment response after high dose melphalan induction therapy at diagnosis and after four cycles of KCd induction before salvage ASCT.
- There was a significant difference between response after upfront ASCT versus response after salvage ASCT (p = 0.04). Upfront and salvage ASCT led to further improvement of response, but this was most pronounced after upfront ASCT where 32.5% achieved complete remission (CR) or stringent CR versus 22.8% achieving CR or stringent CR after salvage ASCT (p = 0.033).
- Previous bortezomib exposure did not affect the quality of response after KCd or after salvage ASCT.
- Toxicities related to induction treatment with KCd mainly consisted of low-grade hematologic AEs and infections, including five cases of septicemia. Cardiac AEs were observed in 22 cases, which in one case led to withdrawal from the study (read more about carfilzomib and cardiovascular AEs in our educational theme here). In addition, three patients died in the induction period due to septicemia (two cases) or progression of MM (one case).
- During the period between salvage ASCT and randomization:
- the most frequent AEs were hematologic events and infections;
- two patients died due to fungus septicemia (100-day mortality of 1.1%); and
- a total of 93 and 57 serious AEs were filed in the induction phase and the time following salvage ASCT, respectively.
- The mean time to neutrophils >1.0 × 109/L after upfront ASCT and salvage ASCT was 16.9 days (standard deviation, 9.4) and 13.4 days (standard deviation, 3.5), respectively (p < 0.0001).
- Median overall survival until data cut-off (September 1, 2019) was not reached in the maintenance group and was 44.5 months (range, 39.8 months to not applicable) in the control group (hazard ratio, 0.47; 95% confidence interval, 0.18–1.19; p = 0.10).
Subgroup analysis
The benefit of carfilzomib-dexamethasone maintenance was observed across prespecified subgroups according to baseline characteristics. Patients randomized to maintenance had the same TTP from the start of induction therapy in the upfront and salvage treatment, namely a median of 31.1 months (range, 29.2–36.8 months) and 31.5 months (range, 29.3 months to not reached), respectively. Improvement of response was observed more often in the maintenance group (52.4%) compared with the control group (34.9%; p = 0.003). Patients in the maintenance group with a partial response at the time of randomization had an 88% response improvement, in comparison to patients in the control group who had a 38% response improvement (p = 0.009).
Safety analysis
The most common AEs recorded following randomization are summarized in Table 2.
Table 2. Incidence of selected adverse events*
|
AVN, avascular necrosis. |
||||||
|
Adverse event, %† |
Carfilzomib-dexamethasone maintenance group |
Control group |
||||
|---|---|---|---|---|---|---|
|
Grade I–II |
Grade III |
Grade IV |
Grade I–II |
Grade III |
Grade IV |
|
|
Hematologic |
|
|
|
|
|
|
|
Anemia |
57 |
1 |
0 |
44 |
0 |
0 |
|
Thrombocytopenia |
29 |
0 |
0 |
21 |
1 |
1 |
|
Neutropenia |
29 |
2 |
1 |
27 |
3 |
1 |
|
Cardiac and pulmonary |
|
|
|
|
|
|
|
Atrial fibrillation |
NA |
1 |
0 |
0 |
1 |
0 |
|
Hypertension |
15 |
4 |
0 |
2 |
1 |
0 |
|
Dyspnea |
21 |
2 |
1 |
10 |
1 |
0 |
|
Bacterial infections |
|
|
|
|
|
|
|
Pneumonia |
2 |
12 |
0 |
0 |
9 |
0 |
|
Other respiratory tract infection |
5 |
6 |
0 |
1 |
6 |
0 |
|
Septicemia |
0 |
0 |
2 |
0 |
0 |
0 |
|
Urinary tract infection |
1 |
1 |
0 |
0 |
3 |
0 |
|
Gastrointestinal tract infections |
0 |
4 |
0 |
0 |
0 |
0 |
|
Fever without focus |
2 |
6 |
0 |
2 |
8 |
0 |
|
Miscellaneous |
1 |
1 |
0 |
2 |
1 |
0 |
|
Steroid-related adverse events |
|
|
|
|
|
|
|
Mood alteration |
20 |
1 |
1 |
6 |
0 |
0 |
|
Fatigue |
12 |
0 |
0 |
7 |
0 |
0 |
|
Insomnia |
12 |
0 |
0 |
0 |
0 |
0 |
|
Pain |
21 |
0 |
0 |
21 |
0 |
0 |
|
Adenocarcinoma |
0 |
1 |
1 |
0 |
0 |
0 |
|
AVN of the femoral head |
0 |
2 |
0 |
0 |
0 |
0 |
|
Thrombosis |
0 |
1 |
0 |
0 |
0 |
0 |
- In total, 53 serious AEs were reported in 30.5% of patients in the maintenance group and 38 serious AEs were reported in 24.4% of the control patients.
- One patient died during maintenance treatment versus none in the control group, and the all-cause Day 100 mortality rate after salvage ASCT was 1.1%.
- AEs led to dose reduction or discontinuation of carfilzomib or dexamethasone in 30.5% of patients in the maintenance group and in 23.3% of patients in the control group.
- There was no significant difference in patient-reported health-related quality of life between the two groups during the follow-up period (95% confidence interval, 1.61–6.09; p = 0.255).
Conclusion
Overall, the use of KCd induction therapy prior to salvage ASCT was feasible and efficacious with manageable toxicity for patients with relapsed MM. Additionally, carfilzomib-dexamethasone maintenance prolonged TTP by 8 months compared with no maintenance treatment. The AEs reported once again open the discussion on whether dexamethasone could be reduced or omitted during maintenance. Limitations of the study include the lack of lenalidomide maintenance after upfront ASCT, which is the current standard of care, as well as the use of TTP instead of PFS as the primary endpoint. Finally, the authors suggest that future efforts should explore the addition of proteasome inhibitors into conditioning regimens with high-dose melphalan.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


 Rakesh Popat
Rakesh Popat