All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Johnson & Johnson, Legend Biotech, Pfizer, Roche, and Sanofi. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Editorial theme | Carfilzomib and cardiovascular adverse events
The field of multiple myeloma (MM) research has progressed rapidly over the recent decade, and the advent of promising novel agents is great news for patients. Newer agents, however, may come with unique sets of side effects and managing these can be a challenge. In our latest editorial theme, the Multiple Myeloma Hub would like to explore how to manage adverse events from novel agents, with our first article examining proteasome inhibitors (PIs) and cardiovascular (CV) events.
CV adverse events
A CV event is an incident that may cause damage to the heart muscle, a state known as cardiotoxicity. CV adverse events include hypertension, arrhythmias, heart failure, and ischemic heart disease. The development of these conditions can have a variable impact on patients depending upon the severity of the condition, with some being managed adequately in an outpatient setting and others requiring intensive monitoring and aggressive treatment.
Patients with MM do experience CV events at a high incidence. The reasons for this are manifold and were covered by Nijhof during the 7th World Congress on Controversies in Multiple Myeloma (COMy).1 The median onset for MM is 70 years old, and as age is a risk factor for CV comorbidities, there is a high baseline within this group for CV events. In addition, MM itself is associated with the development of CV complications, such as vascular thromboembolism (VTE) with an up to 28-fold increase. The risk of VTE is dynamic and changes at different stages, from diagnosis to start of treatment, remission, and through to relapse. Many elderly patients may also have renal issues, which can exacerbate CV events like chronic kidney disease.
Treatment for MM can also impact a patient’s risk of developing CV events. Immunomodulatory agents such as thalidomide have been recorded to increase the risk of VTE 2.6-fold when used alone and 8-fold when given with dexamethasone.
PI toxicity1
All PIs are associated with heart failure; however, the effect is most pronounced with carfilzomib because it binds irreversibly to its target. Cardiotoxicity is frequently recorded in patients with MM treated with carfilzomib. Up to 22% may experience a CV adverse event (any grade), with arrhythmias being the most common (13.3%), followed by hypertension (12.2%), heart failure (4.1%), and ischemic heart disease (1.8%).
CV events often occur after the first few doses of PIs. A history of CV comorbidities makes patients more likely to develop a CV event following carfilzomib treatment.
Suggested mechanism for PI-induced CV adverse events
A mechanism for PI-mediated CV events was described by Ajai Chari and Hajje.2 Endothelial nitric oxide synthase (eNOS) regulation involves the proteasome at multiple stages, including transcription, phosphorylation, and degradation. Decreased bioavailability of NO causes impaired vasodilation and endothelial dysfunction. In addition, misregulation of NO homeostasis has been linked to a host of CV issues, including hypertension, congestive heart failure, coronary artery disease, and other issues such as chronic renal impairment.
PIs appear to act on eNOS in a biphasic, dose-dependent manner. At low concentrations, they may increase eNOS activity by boosting transcription. High doses of PIs cause the accumulation of ubiquitinated protein phosphatase-2A, resulting in lower eNOS activity.
While bortezomib has been associated occasisionally with reports of cardiac dysfunction, these adverse events were only recorded after patients achieved a cumulative dose of ≥20.8 mg/m2. As bortezomib may cause a dose-limiting neuropathy it is possible this prevents patients from experiencing CV adverse events as they infrequently reach the required cumulative dose.
Incidence of CV adverse events
The incidence of CV adverse events in clinical trials with carfilzomib treatment was assessed by Chintan Shah and colleagues in a review and meta-analysis.3
In this study, they found that:
- The cumulative incidence of cardiotoxicity (all grade) for the 2,660 patients included was 8.68% (95% confidence interval [CI], 6.13–11.59%).
- With respect to high-grade cardiotoxicity, (n = 2,808) the cumulative incidence was 4.92% (95% CI, 3.91–6.02%).
- The CV adverse events reported included congestive heart failure, arrhythmias, ischemic heart disease, and cardiorespiratory arrest. The incidence of mortality following a cardiac adverse event was 0.91% (95% CI, 0.49–1.43%).
- Hypertension was also assessed in this meta-analysis, the incidence (all grade) was found to be 11.53% (95% CI, 7.69–15.97%).
- For high-grade hypertension, the incidence was 4.60% (95% CI, 2.42–7.33%) which was calculated with data from 1,981 patients.
To see if the data from clinical trials differ from real-world experience, especially in the older population, Bishnoi, et al. analyzed for carfilzomib-associated adverse events of the Surveillance, Epidemiology, and End Results (SEER)-Medicare data set.4
In a study that included 7,330 patients with MM aged ≥65 years, 11.1% were treated with carfilzomib. Of these, 57.6% had one or more CV adverse events. In this study, CV events were grouped into 4 categories: ischemic heart disease, conduction disorders, hypertension, and heart failure, the results are shown in Table 1. Treatment with carfilzomib was significantly associated with an increased risk (hazard ratio [HR] > 1.00) of experiencing a CV adverse event of all categories except conduction disorders. Also, the age of the patients in this study may be a reason for the increased incidence of CV adverse events.
Table 1. Risk of developing CV adverse events in carfilzomib treated group*
|
CI, confidence interval; HR, hazard ratio. |
||||||
|
Category |
Adverse events in carfilzomib group |
Median time, months |
HR |
95% CI |
p-value |
|
|---|---|---|---|---|---|---|
|
n |
% |
|||||
|
Ischemic heart disease |
143 |
17.6 |
5.2 |
1.45 |
1.19–1.75 |
0.0002 |
|
Heart failure |
132 |
16.2 |
4.3 |
1.47 |
1.20–1.81 |
0.0002 |
|
Conduction disorders and arrhythmias |
214 |
26.3 |
3.6 |
0.95 |
0.82–1.12 |
0.5498 |
|
Hypertension |
225 |
27.6 |
8.2 |
3.33 |
2.83–3.92 |
<0.0001 |
Clinical management of CV events
Patient selection
The European Myeloma Network and the Italian Society of Arterial Hypertension have published guidelines for preventing, monitoring, and treating CV adverse events with carfilzomib.5 Baseline assessment of CV risk factors and a history of CV disease is necessary before starting carfilzomib therapy. A suggested flowchart for patient selection and evaluation prior to starting carfilzomib therapy is shown in Figure 1.
Figure 1. A flowchart for selection and evaluation of patients prior to and during carfilzomib treatment*
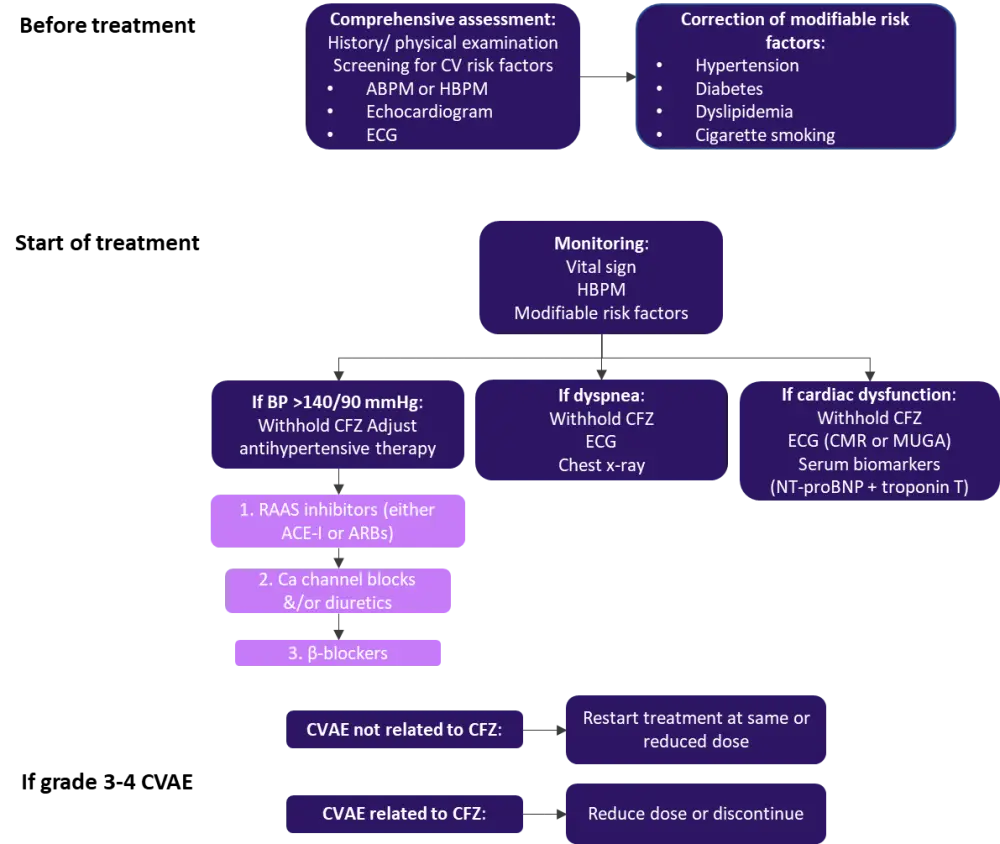
ABPM, ambulatory blood pressure monitoring; ACE-I, angiotensin converting enzyme-inhibitor; ARBs, angiotensin receptor blocker; BP, blood pressure; CFZ, carfilzomib; CVAE, cardiovascular adverse event; ECG, electrocardiogram; HBPM, home blood pressure monitoring; MUGA, multigated angiography; NT-proBNP, N-terminal-pro hormone brain natriuretic peptide; RAAS, renin-angiotensin aldosterone.
*Adapted from Bringhen, et al.5
As hypertension is one of the most frequent CV adverse events, blood pressure monitoring is advised whether in the clinic or at home. Estimation of CV risk should be performed using either the Systematic COronary Risk Evaluation (SCORE) or a table with detailed stratification.
Imaging
Measuring cardiac biomarkers is not essential for the early detection of cardiotoxicity. On the other hand, imaging is integral to CV risk stratification, requiring both structural and functional assessment.
Echocardiography can be used to measure left ventricular ejection fraction (LVEF), which is an important indicator of cardiotoxicity. Cardiotoxicity is diagnosed if a patient has a decrease in LVEF of >10% to >5% accompanied by the symptoms of heart failure. LVEF prior to chemotherapy may also predict risk of cardiotoxicity.
Another parameter that can be useful to measure is global longitudinal strain (GLS), which can be assessed using automated speckle tracking echocardiography. GLS measures the lengthwise contraction of the myocardium and is thought to provide more consistent results than measuring radial or circumferential deformation in analyzing myocardial damage, predicting late onset of cardiotoxicity, and planning cardio-protective strategies. The American Society of Echocardiography (ASE) and European Association of Cardiovascular Imaging (EACVI) consensus states that while a GLS decrease of <8% from the baseline is not meaningful, while >15% is an indicator of pathology.
Cardiac magnetic resonance imaging (MRI) is only necessary when echocardiography results are sub-standard. In addition, cardiac MRI is recommended if there is a LVEF <53%.
Multigated angiography is for patients with sub-optimal echocardiography and cardiac MRI as this technique results in exposing the patient to radiation.
CV adverse event prophylaxis
Chronic over stimulation of the sympathetic nervous system is involved in heart failure; therefore, β-blockers are advised on patients with a decreased LVEF. Angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers (ARBs) can be helpful in combination with β-blockers in preventing cardiotoxicity.
- If a patient has no CV risks and normal blood pressure, they can be started on carfilzomib immediately.
- For patients with MM that have any underlying CV morbidities should be managed before commencing PI treatment.
During therapy
Fluid therapy
For most patients, 250mL fluids infusion prior to the first cycle of carfilzomib is enough to protect or decrease any acute renal impairment. Patients should be monitored for signs or symptoms of fluid overload.
Dyspnea
Fluid overload or drug toxicity are potential causes of dyspnea. For severely dyspneic patients, cessation of carfilzomib treatment is advised until symptoms disappear. EF impairment or other evidence of heart dysfunction are not commonly seen in patients with heart disease who present as dyspneic. These patients can restart their carfilzomib treatment once their symptoms improve.
Blood pressure
Patients blood pressure should be regularly monitored during carfilzomib therapy. Patients are recommended to monitor their blood pressure at home. If a blood pressure of >135/85 mmHg is recorded twice, carfilzomib should be paused and antihypertensive medication should be given until the patients blood pressure returns to a normal range.
Cardiac dysfunction
If a patient develops a CV adverse event, carfilzomib treatment should be paused until the patient recovers and their CV health should be assessed. When the patient recovers, treatment can be resumed, though it is advisable to reduce the dose or change to a bortezomib regimen.
For patients with Grade ≥2 cardiac dysfunction, carfilzomib treatment should be halted, and the following tests performed:
- ECG and echocardiogram with echocardiography-derived strain.
- Cardiac MRI or multigated angiography if no echocardiogram available/ poor image quality.
- Measure cardiac biomarkers in the serum.
- Depending on the results of these tests a cardiologist’s referral may be required.
If cardiac function recovers to baseline or Grade 1 then the continuation of MM therapy should be decided by a hematologist and cardiologist, after evaluating the risk of further cardiac dysfunction against the benefit of continuing MM treatment.
Whether carfilzomib treatment is the reason for the CV adverse event should always be assessed. If a patient experiences a Grade 3/4 treatment-related CV adverse event, a dose reduction or even discontinuation may be required. If the CV adverse event was found to be unrelated to treatment with carfilzomib, therapy can be restarted at an equal or lower dose than used previously.
Conclusion
While carfilzomib is an effective agent for the treatment of MM it is also associated with an increased incidence of CV adverse events. Selecting patients for whom the risk-benefit ratio is positive is crucial, as is managing any modifiable risk factors. Once patients are on carfilzomib, an effective strategy to manage and mitigate these side effects is necessary. Further investigation into the mechanism of action is warranted to improve strategies aimed at reducing CV adverse events with carfilzomib treatment.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

