All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
CAR-T versus bispecific antibodies and BiTEs in multiple myeloma
Chimeric antigen receptor T cells (CAR-T) and bispecific antibodies (bsAbs)/bispecific T-cell engagers (BiTEs), even though they present structural similarities, differ in their mode of action. CAR-T therapies rely on ex vivo activated and expanded T cells, whereas bsAbs/BiTEs count on the patient’s endogenous T cells.
During the 3rd European CAR T-cell Meeting, Sébastien Anguille discussed some true and false statements about CAR-T and bsAbs/BiTEs in multiple myeloma (MM).1 Test your knowledge below and review some of the most critical aspects when comparing these two cutting-edge therapies.
Poll 1
Your opinion matters
CAR-T products are more advanced than bsAbs/BiTEs in MM, and BCMA is the most popular target antigen
There are at least 23 different B-cell maturation antigen (BCMA)-targeted CAR-T products and seven different bsAB/BiTEs directed to BCMA in clinical development.1
The three most advanced BCMA CAR-T products are ide-cel, cilta-cel, and orva-cel; ide-cel being the first CAR-T in MM approved by the FDA. The BCMA-directed bsAbs/BiTEs are still in phase I clinical trials.1
This statement is definitely true.
Their administration is also different: CAR T cells are usually administered as a single intravenous infusion, whereas most of the bsAbs/BiTEs, due to their very short half-life, need to be administered intravenously or subcutaneously once weekly.1
Poll 2
Your opinion matters
bsAbs/BiTEs have a more favorable safety profile than CAR-T products
Of 639 patients treated with the 23 different BCMA CAR-Ts, 80.3% experienced cytokine release syndrome (CRS) of any grade and 14.1% of Grade ≥3; whereas of the 359 patients treated with the seven different BCMA bsAbs/BiTEs, 55.2% experienced CRS of any grade, and only 2.8% had Grade ≥3 CRS.2
Table 1 shows the percentage of patients experiencing CRS and neurotoxicity, the median time of onset, and the median duration of CRS with BCMA CAR-T products and BCMA bsAbs/BiTEs. Patients treated with bsAbs/BiTEs experienced CRS events earlier, but they were less long-lasting than those experienced with CAR-T.1 Besides, even though the administration of bsAbs/BiTEs is not a one-time event (unlike CAR-T), CRS events usually occur in the first administration. As observed for CRS, neurotoxicity is also less frequent with bsAbs/BiTEs, except for PF-3135, which shows a neurotoxicity rate comparable to that observed with CAR-T (20%).1
Fewer CRS and neurotoxicity events were observed with bsAbs/BiTEs; hence, to date, this statement is true.
Table 1. CRS and neurotoxicity with BCMA CAR-T and bsAbs/BiTEs1
|
BiTE, bispecific T-cell engager; bsAb, bispecific antibodies; CAR, chimeric antigen receptor; CRS, cytokine release syndrome; d, day; hr, hour; ND, not determined; NT, neurotoxicity. |
||||||||||
|
|
CAR-T |
bsAbs/BiTEs |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
Ide-cel |
Cilta-cel |
Orva-cel |
AMG420 |
AMG701 |
REGN 5458 |
TNB-383B |
CC-93269 |
PF-3135 |
Teclistamab |
|
|
N |
128 |
97 |
62 |
42 |
85 |
49 |
58 |
30 |
30 |
n = 65* |
|
CRS |
84% |
95% |
89% |
38% |
65% |
39% |
45% |
77% |
73% |
57% |
|
Median time of onset, (range) |
1 d |
7 d |
2 d |
ND |
ND |
18 hr |
<24 hr |
24 hr |
<48 hr |
48 hr |
|
Median duration, d (range) |
5 |
4 |
4 |
ND |
ND |
0.5 |
1 |
2 |
2 |
2 |
|
NT |
18% |
21% |
13% |
7% |
ND |
12% |
2% |
0 |
20% |
5% (N = 149) |
Poll 3
Your opinion matters
CAR-T outperform bsAbs/BiTEs in terms of response rates
A systematic review and meta-analysis of the BCMA CAR-T clinical trials showed that of 630 treated patients, 80.5% had an objective response, with 44.8% achieving a complete response (CR).2 Among the BCMA CAR-T products, cilta-cel showed the highest CR rates (67% vs 33% for ide-cel vs 36% for orva-cel).1 Efficacy reported with BCMA bsAbs/BiTEs is lower and less deep, with 41.4% of 345 treated patients demonstrating an objective response (Figure 1).1
Figure 1. Response rates reported with BCMA CAR-T and bsAbs/BiTEs1
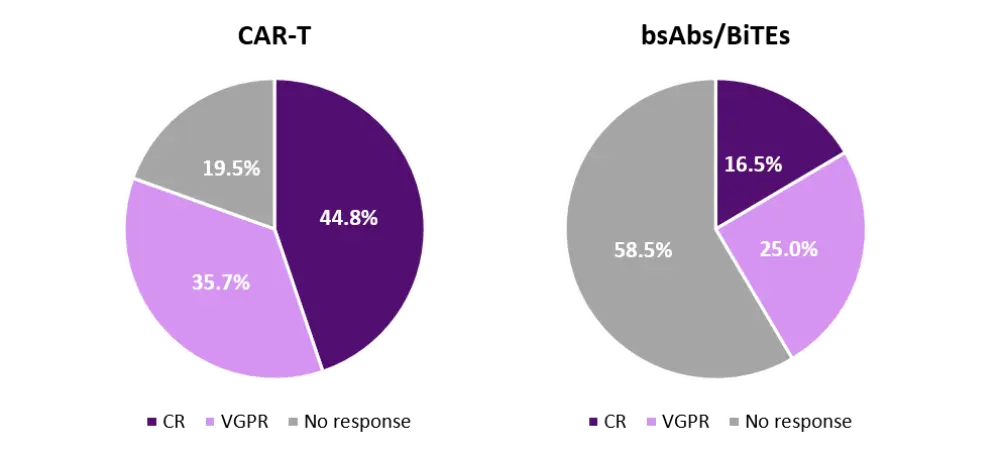
BiTE, bispecific T-cell engager; bsAb, bispecific antibody; CAR-T, chimeric antigen receptor T cells; CR, complete response; VGPR, very good partial response.
It is important to note that BCMA bsAbs/BiTEs have been evaluated mainly in phase I dose-finding clinical trials, and some patients were treated with suboptimal doses. When analyzing separately patients treated at top dose level or with the recommended phase II dose (n = 93), the objective response rate increases to 75%, with 26% of patients achieving a CR, 33% a very good partial response, and 16% a partial response (Figure 2). Here, it is possible to appreciate a similar efficacy between CAR-T and bsAbs/BiTEs, although this will need confirmation in future phase II trials. Thus, to date, this statement is false. They show similar ORRs in advanced MM.
Figure 2. Response rates in patients treated with top doses or at the recommended phase II dose1
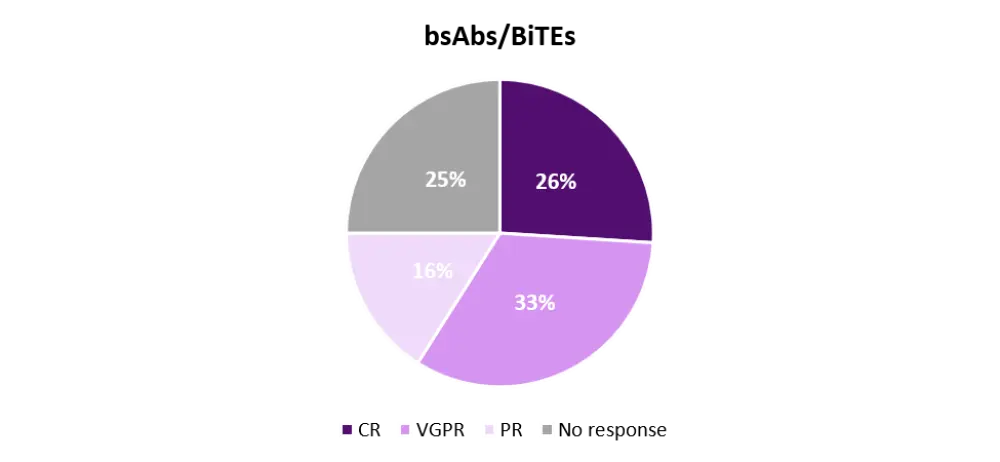
BiTE, bispecific T-cell engager; bsAb, bispecific antibody; CR, complete response; PR, partial response; VGPR, very good partial response.
Conclusion
- CAR-T and bsAbs/BiTEs share similarities but they constitute different therapeutic classes
- BCMA is the most popular target antigen, both in the context of CAR T-cell therapy as well as in the context of bsAbs/BiTEs
- CAR T-cell therapies are more advanced for the treatment of MM than bsAbs/BiTEs
- bsAbs/BiTEs seem to have a more favorable safety profile in terms of CRS events and neurotoxicity
- Although these are indirect comparisons, bsAbs/BiTEs seem to have an efficacy comparable to CAR-T in the relapsed/refractory setting
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?

