All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
EVOLVE Trial: Can innovative orva-cel technology be a new option for patients with relapsed/refractory MM?
Orvacabtagene autoleucel (orva-cel) is under investigation as a B-cell maturation antigen (BCMA)-directed chimeric antigen receptor (CAR) T-cell product with a fully human binder, and it has shown in vitro activity even in low-BCMA cells. The optimized construct reduces antigen-independent exhaustion by minimizing tonic signaling and improving binding to BCMA on target cells. A novel manufacturing process has allowed for a purified CD4+ and CD8+ CAR T-cell product enriched for a central memory phenotype, with promising persistence and durability.
EVOLVE (NCT03430011) is a phase I/II study investigating different dose ranges of CAR T cells in patients with relapsed/refractory multiple myeloma (RRMM). The previously published results for lower doses (50 × 106 and 150 × 106) have demonstrated an acceptable safety profile with promising clinical activity. 1 Recently, the study has reached two primary objectives for phase I that have been covered in this article. Sham Mailankody and colleagues shared their results from patients treated with higher doses during the American Society of Clinical Oncology (ASCO) 2020 Virtual Annual Meeting.2
Study design2
Patients were deemed eligible if they met the following criteria:
- RRMM
- Received at least three prior lines of therapy, including autologous stem cell transplant, immunomodulatory drugs, proteasome inhibitors, and an anti-CD38 agent
- Refractory to the last line of therapy
- Eastern Cooperative Oncology Group (ECOG) performance status of 0–1
- Creatinine clearance ≥ 60 mL/min, absolute neutrophil count ≥ 1000 cells/mm3, platelets ≥ 50/mm6, hemoglobin ≥ 8 g/dL
Selection process was independent of BCMA expression, but a BCMA cohort was defined with patients relapsed following prior anti-BCMA therapy.
For phase I, primary objectives included safety evaluation and identification of a recommended phase II dose. Secondary objectives included the determination of pharmacokinetics and preliminary antitumor activity.
Methodology
- Lymphodepletion with fludarabine 30 mg/m2 and cyclophosphamide 300 mg/m2 for 3 days
- Orva-cel therapy at the doses of 300 × 106, 450 × 106, and 600 × 106 CAR T cells, manufactured using the process intended for commercial use
- Bone marrow examination at Day 15 and disease assessment at Day 29
- Posttreatment follow-up up to 24 months; long-term monitoring up to 15 years
Patient characteristics
A total of 62 patients were included in the study. The median age was 61 years (range, 33–77), and was similar among the three dose groups. The median time from myeloma diagnosis was 7 years (range, 2–24). The proportion of patients with extramedullary plasmacytomas was 23%. Nearly half of the patients had International Staging System (ISS) stage I disease. The median number of prior therapies was 6 (range, 3–18), and all patients were refractory to their last regimen. Baseline characteristics are summarized in Table 1 below.
Table 1. Baseline characteristics among three dose groups2
|
auto-SCT, autologous stem cell transplant; IMiD, immunomodulatory drug; PI proteasome inhibitor |
||||
|
Characteristic, n (%) |
Dose group (CAR T cells) |
|||
|---|---|---|---|---|
|
300 × 106 (n = 19) |
450 × 106 (n = 19) |
600 × 106 (n = 24) |
Total (N = 62) |
|
|
High-risk cytogenetics |
8 (42) |
8 (44) |
9 (38) |
25 (41) |
|
Prior auto-SCT ≥ 2 ASCT |
19 (100) 7 (37) |
16 (84) 1 (5) |
23 (96) 3 (13) |
58 (94) 11 (18) |
|
Penta-exposed (2 IMiDs®, 2 PIs, and anti-CD38 agent) |
17 (89) |
18 (95) |
23 (96) |
58 (94) |
|
Triple-refractory (IMiD, PI, and anti-CD38 agent) |
19 (100) |
18 (95) |
21 (88) |
58 (94) |
|
Penta-refractory (2 IMiDs, 2 PIs, and anti-CD38 agent) |
10 (53) |
11 (58) |
9 (38) |
30 (48) |
|
Received bridging chemotherapy |
9 (47) |
13 (68) |
17 (71) |
39 (63) |
|
Refractory to bridging chemotherapy |
6 (75) |
11 (85) |
11 (65) |
28 (74) |
Results2
- The most common adverse events were hematologic toxicities and cytokine release syndrome (CRS; any grade), with similar frequency across three dose groups
- Grade ≥ 3 CRS was low (3%)
- Neutropenia was the most common type of any grade cytopenia, occurring in 90% of patients
- No bleeding events were related to thrombocytopenia
- The incidence of all Grade ≥ 3 infections was 13%
Grade ≥ 3 adverse events and events of special interest are summarized in Table 2 and Table 3, respectively.
Table 2. Overall incidence of Grade ≥ 3 adverse events2
|
AE, adverse event |
|
|
AE, n (%) |
Grade ≥ 3 AEs (N = 62) |
|---|---|
|
Neutropenia |
56 (90) |
|
Anemia |
30 (48) |
|
Thrombocytopenia |
29 (47) |
|
Leukopenia |
20 (32) |
|
Hypophosphatemia |
13 (21) |
|
All infections |
8 (13) |
Two deaths occurred within 90 days following orva-cel infusion: one due to comorbidities in the 300 × 106 CAR T cells group (considered not to be related to orva-cel infusion) and one due to a Grade 5 event in the 450 × 106 CAR T cells group (macrophage activation syndrome/hemophagocytic lymphohistiocytosis, considered to be related to orva-cel infusion). A Grade 3 neurologic event (NE) and Grade 4 neutropenia were dose-limiting toxicities reported in two patients (one in the 300 × 106 CAR T cells group and one in the 450 × 106 CAR T cells group).
Table 3. Summary of safety findings by orva-cel dose2
|
AE, adverse event; CRS, cytokine release syndrome; HLH, hemophagocytic lymphohistiocytosis; MAS, macrophage activation syndrome; NE, neurologic event; SAE, serious adverse event |
|||
|
AEs, n (%) |
Dose group (CAR T cells) |
||
|---|---|---|---|
|
300 × 106 (n = 19) |
450 × 106 (n = 19) |
600 × 106 (n = 24) |
|
|
Any SAE |
4 (21) |
5 (26) |
8 (33) |
|
Grade ≥ 3 events of special interest |
|||
|
Neutropenia |
15 (79) |
19 (100) |
22 (92) |
|
Anemia |
8 (42) |
8 (42) |
14 (58) |
|
Thrombocytopenia |
6 (32) |
10 (53) |
13 (54) |
|
All infections |
3 (16) |
4 (21) |
1 (4) |
|
CRS |
0 |
1 (5) |
1 (4) |
|
NE |
1 (5) |
1 (5) |
0 |
|
MAS/HLH |
0 |
2 (11) |
1 (4) |
CRS and/or NE events were treated with tocilizumab (76%), steroids (52%), the combination of tocilizumab and steroids (50%), anakinra (23%), and siltuximab (3%). Both AEs resolved in a median of 4 days (range, 1–10).
The objective response rate was 92% for all dose groups, and 68% of patients reached at least a very good partial response (see Figure 1). Of all evaluable patients, 84% were measurable (minimal) residual disease (MRD) negative at 1 × 10-5 depth at Month 3. Six months after infusion, persistent CAR T cells were detected in 69% of evaluable patients.
Figure 1. Response rates across all dose groups2
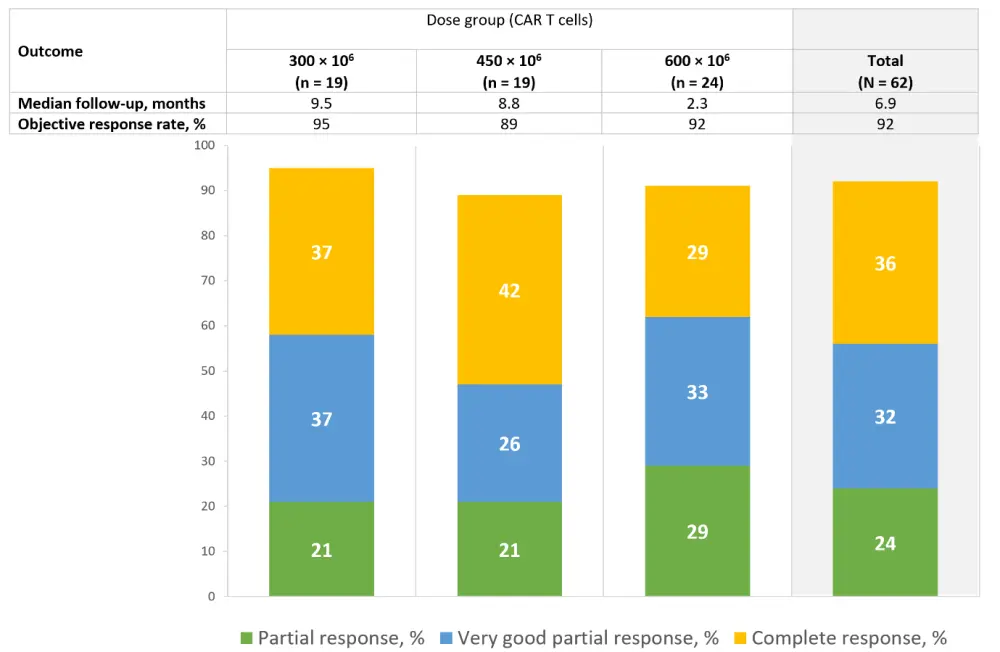
Deep serologic responses were seen in all patients at doses of 450 × 106 and 600 × 106 CAR T cells. The investigators highlighted these as relevant results, considering the median follow-up of 6.9 months and that this was achieved in all groups. Median progression-free survival was 9.5 months in the 300 × 106 CAR T cells dose group while it was not reached at doses of 450 × 106 and 600 × 106 CAR T cells. All patients with high baseline soluble BCMA responded to the treatment; moreover, 67% of them achieved a very good partial response or better.
Conclusion
Orva-cel infusion is an innovative therapy, with an improved manufacturing process enriching for central memory T cells. The safety profile was promising and manageable, with low incidence of Grade ≥ 3 CRS and NE in all dose groups. Orva-cel infusion with higher dose levels was associated with high response rates, and robust expansion was observed in all dose groups in patients who previously received several lines of therapy. The phase II part of the study is still ongoing, and updated results will be presented in due course.
Learn more about trials with CAR T-cell therapies in multiple myeloma here.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?

