All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Johnson & Johnson, Legend Biotech, Pfizer, Roche, and Sanofi. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
BCMA-directed immunotherapies in MM: The importance of sequencing
Do you know... Which of these factors do NOT influence sequencing strategies for the treatment of adults with multiple myeloma?
B-cell maturation antigen (BCMA)-targeted therapies have demonstrated promising efficacy in the treatment of relapsed/refractory multiple myeloma (MM).1 However, the sequencing of these therapies remains controversial, with debate centered around the optimal order of administration to maximize patient outcomes while considering factors like resistance mechanisms, toxicities, and treatment accessibility.2
BCMA-directed immunotherapies
BCMA is a cell surface antigen that is heavily involved in the survival and proliferation of plasma cells by interacting with its ligands, B-cell activating factor (BAFF), and A proliferation-inducing ligand (APRIL).1 BCMA is an optimal target for the treatment of MM due to its high expression on malignant cells and relatively low expression on other tissues, therefore minimizing off-target effects of treatment.1
There are a number of T-cell-engaging (TCE) therapies that target BCMA for the treatment of MM. These include antibody-drug conjugates, as well as modern therapies including chimeric antigen receptor (CAR) T cells and bispecific antibodies (bsAbs) (Figure 1).3
Figure 1. Mechanism of action for T-cell engagers*
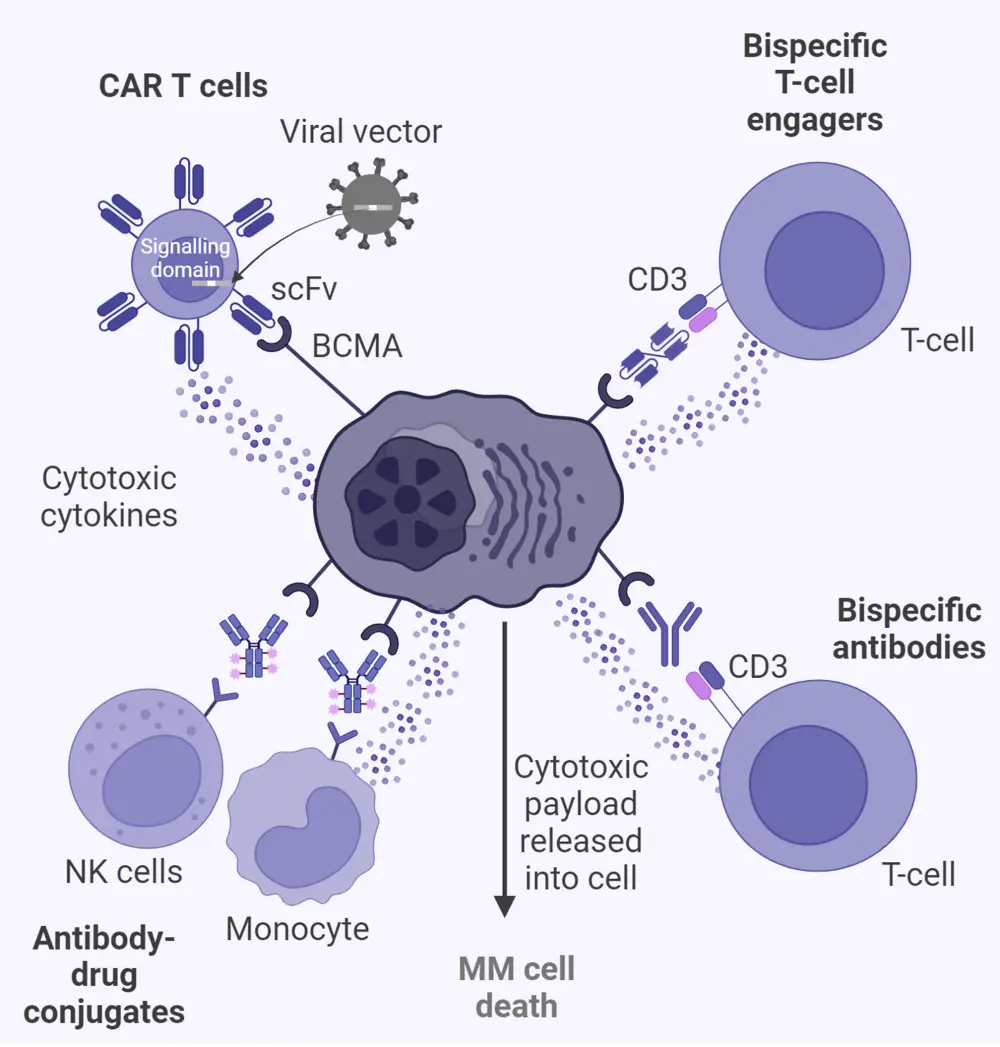
Created with BioRender.com.
Ab, antibody; BCMA, B-cell maturation antigen; BM, bone marrow; CAR, chimeric antigen receptor; FcRH5, Fc receptor-homolog 5; GC, germinal center; GPRC5D, G-protein–coupled receptor class C group 5 member D; IFN, interferon; IL, interleukin; MM, multiple myeloma; NK, natural killer; PC, plasma cell; RR, relapsed/refractory; scFv, single-chain fragment variable; SLAM, signaling lymphocytic activation molecule; TNF, tumor necrosis factor.
*Adapted from Cho, et al.3 under the Creative Commons Attribution 4.0 International License.
BCMA is a member of the tumor necrosis factor receptor (TNFR) superfamily and primarily binds to BAFF and APRIL. In MM, bone marrow stromal cells (BMSCs) are a primary source of BAFF and APRIL.4 These cells create a supportive microenvironment for myeloma cells within the bone marrow. In addition, macrophages and osteoclasts within the bone marrow microenvironment also play significant roles in the secretion of BAFF and APRIL, contributing to the survival, proliferation, and resistance of myeloma cells. Myeloma cells themselves can also secrete BAFF and APRIL, creating an autocrine loop that further supports their own growth and survival.4
The binding of these ligands to BCMA triggers a series of intracellular signaling cascades.4 The activation of these pathways by BCMA supports the proliferation of plasma cells by promoting gene expression that prevents apoptosis and enhances cell division of malignant cells. The disruption of BCMA signaling aims to induce apoptosis and inhibit the proliferation of myeloma cells (Figure 2).4
Figure 2. BCMA signaling pathway*
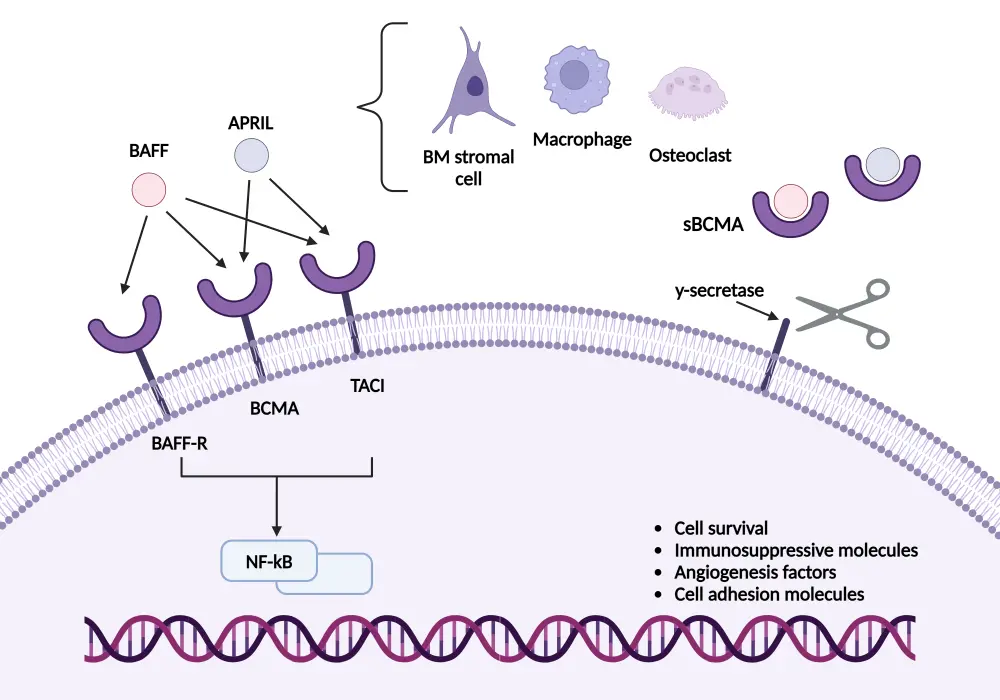
APRIL, A proliferation-inducing ligand; BAFF, B-cell activating factor; BCMA, B-cell maturation antigen; BM, bone marrow; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; TACI, transmembrane activator and CAML interactor.
Created with BioRender.com.
*Adapted from Yu, et al.4 under the Creative Commons Attribution 4.0 International License.
There are a number of approved TCEs for the treatment of MM (Table 1). Bispecific antibody and CAR T-cell therapies were initially investigated in the relapsed/refractory (RRMM) setting, primarily in the heavily pretreated population. However, TCEs are increasingly being investigated in earlier lines of therapy, including in the first line.5,6
Table 1. Currently approved BCMA-directed TCEs in RRMM*
|
CAR, chimeric antigen receptor; IMiD, immunomodulatory agent; mAb, monoclonal antibody; PI, proteosome inhibitor; RRMM, relapsed/refractory multiple myeloma; TCE, T-cell engager. |
|||||
|
Agent |
Category |
FDA approval Date |
FDA indication |
EU approval date |
EU indication |
|---|---|---|---|---|---|
|
Idecabtagene vicleucel |
CAR T-cell therapy |
March 20217 |
After ≥4 prior lines of therapy, including a PI, an IMiD, and an anti-CD38 mAb7 |
August 20218 |
After ≥3 prior lines of therapy, including a PI, an IMiD, and an anti-CD38 mAb, with progression after the last therapy8 |
|
Ciltacabtagene autoleucel |
CAR T-cell therapy |
February 20229 |
After ≥4 prior lines of therapy, including a PI, an IMiD, and an anti-CD38 mAb9 |
May 202210 |
After ≥3 prior lines of therapy, including a PI, an IMiD, and an anti-CD38 mAb, with progression after the last therapy10 |
|
Teclistamab |
Bispecific antibody |
October 202211 |
After ≥4 prior lines of therapy, including a PI, an IMiD, and an anti-CD38 mAb11 |
August 202212 |
After ≥3 prior lines of therapy, including a PI, an IMiD, and an anti-CD38 mAb, with progression after the last therapy12 |
|
Elranatamab |
Bispecific antibody |
August 202313 |
After ≥4 prior lines of therapy, including a PI, an IMiD, and an anti-CD38 mAb13 |
December 202314 |
After ≥3 prior lines of therapy, including a PI, an IMiD, and an anti-CD38 mAb, with progression after the last therapy14 |
Resistance to BCMA-directed therapies15
Although BCMA is considered an optimal target for therapy, resistance is common and can arise through several mechanisms. The primary mechanism of resistance to BCMA is as a result of the downregulation or loss of BCMA expression on the surface of myeloma cells. Genetic mutation or deletions in the TNFRSF17 gene, which encodes BCMA, can reduce antigen expression, making BCMA-directed therapies less effective.15 Antigen escape is a notable mechanism of resistance where malignant cells downregulate antigen expression under therapeutic pressure. This can occur through selective pressure favoring clonal evolution and expansion of BCMA-negative or low-expressing cells15 (Figure 3). T-cell exhaustion further contributes to resistance to BCMA-directed therapies. The persistent stimulation of T cells by tumor antigens, paired with the immunosuppressive environment within the bone marrow leads to T-cell exhaustion and reduced efficacy of BCMA-directed therapies (Figure 3).16
Figure 3. Impact of T-cell exhaustion and BCMA loss on CAR T-cell action*
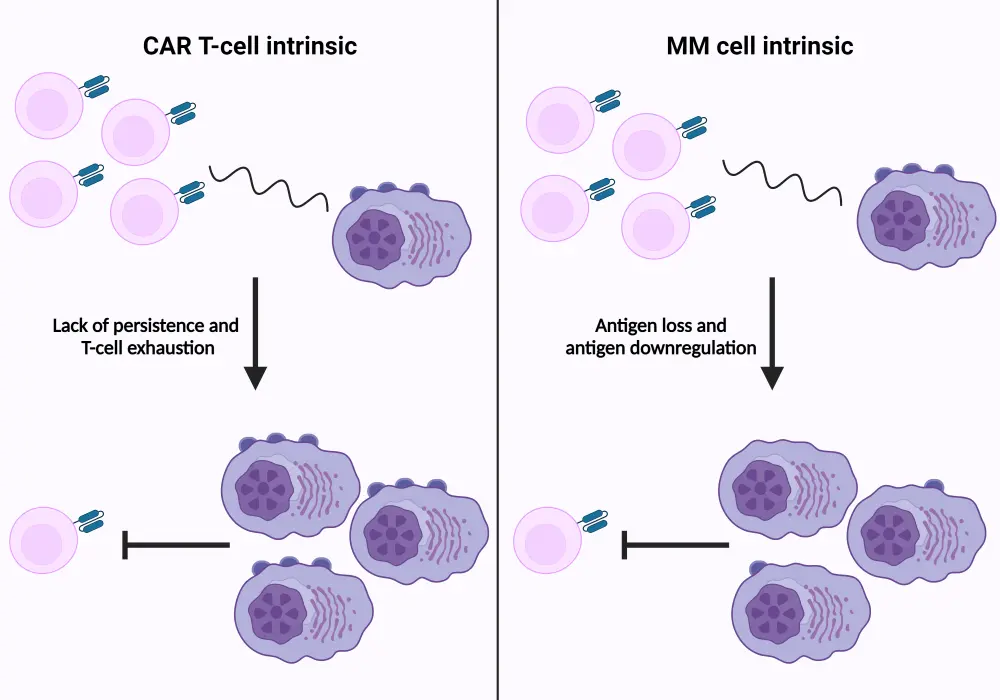
CAR, chimeric antigen receptor; MM, multiple myeloma.
*Adapted from Lee, et al.15 and D’Agostino and Raje.16
The need for novel approaches in RRMM
Despite continual advances in the treatment of MM, many patients still go on to experience disease progression and relapse, highlighting an unmet need for more durable and effective treatment approaches in this setting.17 There is increasing heterogeneity amongst patients with RRMM due to complex treatment histories and resistance to therapies. The prognosis for patients with RRMM is often poor, with patients commonly experiencing diminished duration of response and higher rates of toxicity with increasing lines of therapy.17 The importance of sequencing therapies, and the development of individualized treatment plans is vital to reduce resistance and achieve an optimal response in the absence of intolerable adverse events.
Sequencing strategies
There are a number of factors which influence the optimal sequencing of BCMA-directed therapies, including:
- The stage of disease and patient factors18:
- Comorbidities and overall health status play a key role in developing individual strategies. Patients with a significant number of comorbidities, or those who are frail, may benefit more from less-aggressive therapies to reduce the risk of developing intolerable toxicities.
- Younger and fitter patients may benefit from early intervention with therapies that have the highest efficacy rates such as CAR T-cell therapy and bispecific antibodies. Logically, the most effective and toxic therapies being delivered in earlier lines is rational to elicit the best response when the patient is more able and potentially willing to tolerate them.
- Prior treatments19:
- Prior treatment history, including responses and acquired toxicities, are important to guide further management strategies. For example, in certain situations, patients who have been previously treated with a BCMA-directed therapy may benefit more from treatments which target an alternative antigen. However, there have also been favorable responses noted when anti-BCMA T-cell engagers are administered after an anti-BCMA CAR T-cell therapy.
- Furthermore, in patients who relapse quickly after directed immunotherapies, there should be a consideration for the possible mechanisms of resistance and potential selection of an alternative target.
- Patients who have relapsed a long time after treatment with a BCMA-directed therapy may still be considered for future BCMA-directed treatment, potentially with an alternative therapy in the interim.
- Availability and access20:
- There should be practical considerations made for the accessibility of certain therapies, such as CAR T-cell therapy, where availability is particularly limited and there are longer manufacturing times.
- However, future directions with CAR T-cell therapies include investigation into academic CAR T with notably shorter manufacturing times.21
- Furthermore, the outpatient administration of ciltacabtagene autoleucel (cilta-cel) is being explored in the CARTITUDE-2 and CARTITUDE-5 studies, which have the potential to increase accessibility to treatment, particularly in those who live significant distances from infusion centers.22,23
- In patients with particularly aggressive disease, it may be more appropriate to select a bispecific antibody that is available off-the-shelf to reduce the risk of mortality during manufacturing time.23
- Certain novel therapies, such as CAR T cells, are not approved globally, with some areas in South America having no approved MM-specific CAR T-cell therapies at all.23
- There should be practical considerations made for the accessibility of certain therapies, such as CAR T-cell therapy, where availability is particularly limited and there are longer manufacturing times.
Clinical trial data to support sequencing strategies in MM
There are a number of clinical trials that have evaluated the effect of sequencing in therapies for RRMM. A significant difference in outcomes based on prior therapies has been observed with BCMA-directed therapies, further emphasizing the need for individualized treatment strategies with consideration for prior therapy.6
As part of the MajesTEC-1 (NCT03145181) clinical trial, patients with prior treatment with BCMA-directed therapies in Cohort C were observed for the influence the type of prior therapy had on response to teclistamab, a BCMA-directed bispecific antibody (Figure 4).24 Ultimately, data determined little difference based on the type of therapy administered.
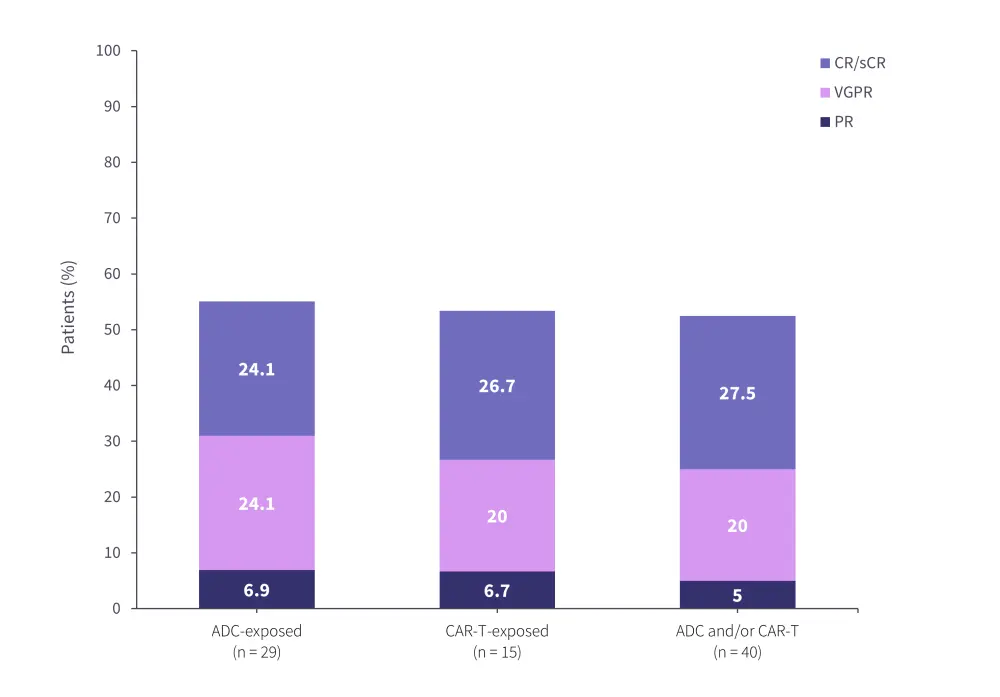
Similarly, in a study of teclistamab in the real-world setting, there was a significant difference in response to therapy based on the nature of the prior therapy received. Patients who had received prior treatment with an antibody-drug conjugate and a CAR T-cell therapy had a higher overall response rate compared with either type of drug alone (Figure 5).25
A difference in progression-free survival (PFS) was observed in Cohort C of the CARTITUDE-2 (NCT04133636) trial of cilta-cel based on prior therapy. CAR T-cell therapy after prior anti-BCMA bispecific antibodies was associated with significantly lower PFS than anti-BCMA-naïve patients at 2.7 months and 8.9 months. This may suggest that CAR T-cell therapies be prioritized over anti-BCMA bispecific antibody, where feasible.26,27
Figure 4. Response rates from cohort C of MajesTEC-1 in patients with prior BCMA‑targeted therapy*
ADC, antibody-drug conjugate; CAR, chimeric antigen receptor; CR, complete response; ORR, overall response rate; PR, partial response; sCR, stringent CR; VGPR, very good PR.
*Data from Raje.24
Figure 5. Real-world teclistamab response rates by prior BCMA-directed therapy*
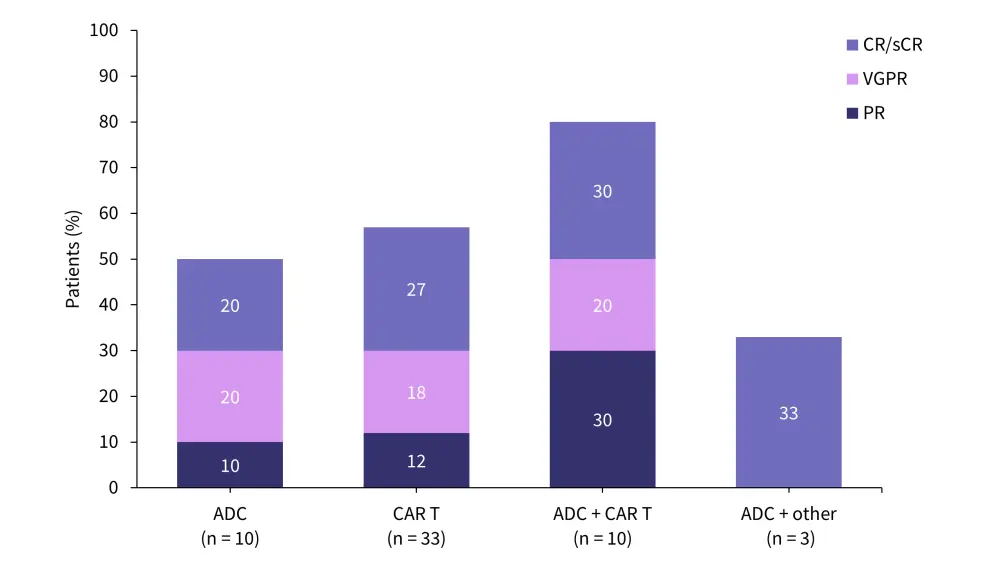
ADC, antibody-drug conjugate; CAR, chimeric antigen receptor; CR, complete response; ORR, overall response rate; sCR, stringent CR; VGPR, very good partial response.
*Data from Dima.25
BCMA-directed therapies in earlier lines of treatment
The implementation of BCMA-directed therapies into earlier lines of therapy is a current point of discussion for the management of MM. The key rationale for investigation into earlier lines are centered around overcoming the mechanisms of resistance, particularly to CAR T cells. This could be achieved due to:
- Presence of fitter T cells in earlier disease
- Having treatment with few prior lines of therapy is associated with improved persistence of CAR T cells, and ultimately increased rates of myeloma cell death
- Increased immunogenicity of tumor cells
- Less pre-treatment heterogeneity in the antigen density expressed on tumor cells results in reduced resistant clone selection
- Lower tumor burden
- Implementation of CAR T cells in earlier lines means tumor cells possess a lower proliferative potential
Additionally, there tends to be lower attrition rates for therapies in later lines; therefore, it may be justified to implement treatments with the highest response rates as early as possible in the treatment paradigm. For example, in Austria, 85% of patients with MM were unable to receive treatment beyond the third line of therapy, with similar data observed in the US, France, and Australia, highlighting the need for effective therapies in early lines where attrition rates remain high.23
Trials investigating BCMA-directed therapies in earlier lines of therapy are outlined in Table 2.
Table 2. Summary of trials investigating BCMA-directed therapies in earlier lines of therapy
|
Auto-HSCT, autologous hematopoietic stem cell transplant; MM, multiple myeloma; RRMM, relapsed/refractory multiple myeloma. |
|||
|
Agent |
Trial |
Phase |
Intervention |
|---|---|---|---|
|
Cilta-cel |
Phase III |
Cilta-cel for the treatment of patients with lenalidomide-refractory MM after 1–3 prior lines of therapy28 |
|
|
Cilta-cel |
CARTITUDE-5 (NCT04923893) |
Phase III |
Bortezomib-lenalidomide-dexamethasone, followed by cilta-cel for the treatment of newly diagnosed MM where transplant is not planned as initial therapy29 |
|
Cilta-cel |
CARTITUDE-6 (NCT05257083) |
Phase III |
Daratumumab-bortezomib-lenalidomide-dexamethasone followed by cilta-cel for the treatment of newly diagnosed MM29 |
|
Ide-cel |
Phase I/II |
Ide-cel in combination with other therapies for the treatment of RRMM with either 1–3 or ≥3 prior regimens30 |
|
|
Ide-cel |
Phase III |
Ide-cel with lenalidomide maintenance for the treatment of newly diagnosed multiple myeloma with suboptimal response after auto-SCT.31 |
|
|
Teclistamab |
Phase III |
Teclistamab-daratumumab-lenalidomide for the treatment of newly diagnosed multiple myeloma, either ineligible for or not intended for auto-HSCT.32 |
|
|
Elranatamab |
MagnetisMM-7 (NCT05317416) |
Phase III |
Elranatamab post-transplant for the treatment of newly diagnosed multiple myeloma.33 |
Future directions
The strategic sequencing of BCMA-directed immunotherapies is vital to improve outcomes in patients with RRMM. Considering the unique mechanisms of action, efficacy, and toxicity profiles of BCMA-directed therapies, as well as patient factors and prior therapies, there is a need to tailor treatment plans to the individual needs of patients. Ongoing research into the optimal sequence and combination strategies for BCMA- and other directed immunotherapies in MM are crucial to maximize the efficacy of treatments, and at the same time limit the associated toxicities.
This educational resource is independently supported by Johnson & Johnson. All content is developed by SES in collaboration with an expert steering committee; funders are allowed no influence on the content of this resource.
Your opinion matters
As a result of this content, I commit to reviewing the latest clinical data to help inform sequencing treatment in my clinical practice.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

