All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Antigen escape as a mechanism of resistance to T-cell-engaging therapies in multiple myeloma
T-cell-engaging therapies function to form an immune synapse between immune effectors and multiple myeloma (MM) cells. The transient durability of clinical response associated with T-cell engagers, and the mechanisms underlying these, are not fully understood. However, antigen escape is one of the key mechanisms that has been linked to resistance to T-cell engagers.1
Here, we summarize a presentation by Paola Neri,1 presented at the 5th Immune Effector Cell Therapies in Multiple Myeloma Workshop on the mechanisms of antigen escape after T-cell-redirecting therapies.
Key points1,2
- Two of the most common targets for immunotherapies in MM are B-cell maturation antigen (BCMA) and G-protein-coupled receptor family C group 5 member D (GPRC5D).
- Amongst both BCMA- and GPRC5D-directed T-cell engagers, clinical effectiveness is often transient, particularly in the relapsed/refractory setting.
- Antigen escape is one of the most prominent mechanisms of acquired resistance to T-cell-engaging therapies.
- Target antigen loss is commonly observed following BCMA- and GPRC5D-directed CAR T-cell and T-cell-engaging therapies (Figure 1).
- A biallelic loss or mutation in TNFRSF17 has been observed in 42% of patients after treatment with T-cell-engaging therapies and 6% after BCMA-directed chimeric antigen receptor (CAR) T cells.
- Amongst patients treated with GPRC5D-directed CAR T cells, a reduction or loss of GPRC5D protein was observed in all six cases who experienced progression after treatment.
- The downregulation of GPRC5D is often observed at the point of relapse after directed CAR T-cell therapy.
- Antigen escape can also be attributed to tumor heterogeneity and selection pressure.2
- Interpatient and intratumor heterogeneity have both been linked to progression and therapy resistance.
- The genomic loss of BCMA is attributed to biallelic TNFRSF17 loss involving pre-existing chromosome 16p loss.
- A monoallelic loss of 16p may be associated with an increased risk of progression after BCMA-directed therapies.
- An epigenetic silencing of GPRC5D has been observed after treatment with talquetamab, associated with:
- Biallelic genetic inactivation of GPRC5D; or
- Long-range epigenetic silencing of its promoter and enhancer regions.
Figure 1. Antigen escape as a mechanism of resistance to CAR T-cell therapy*
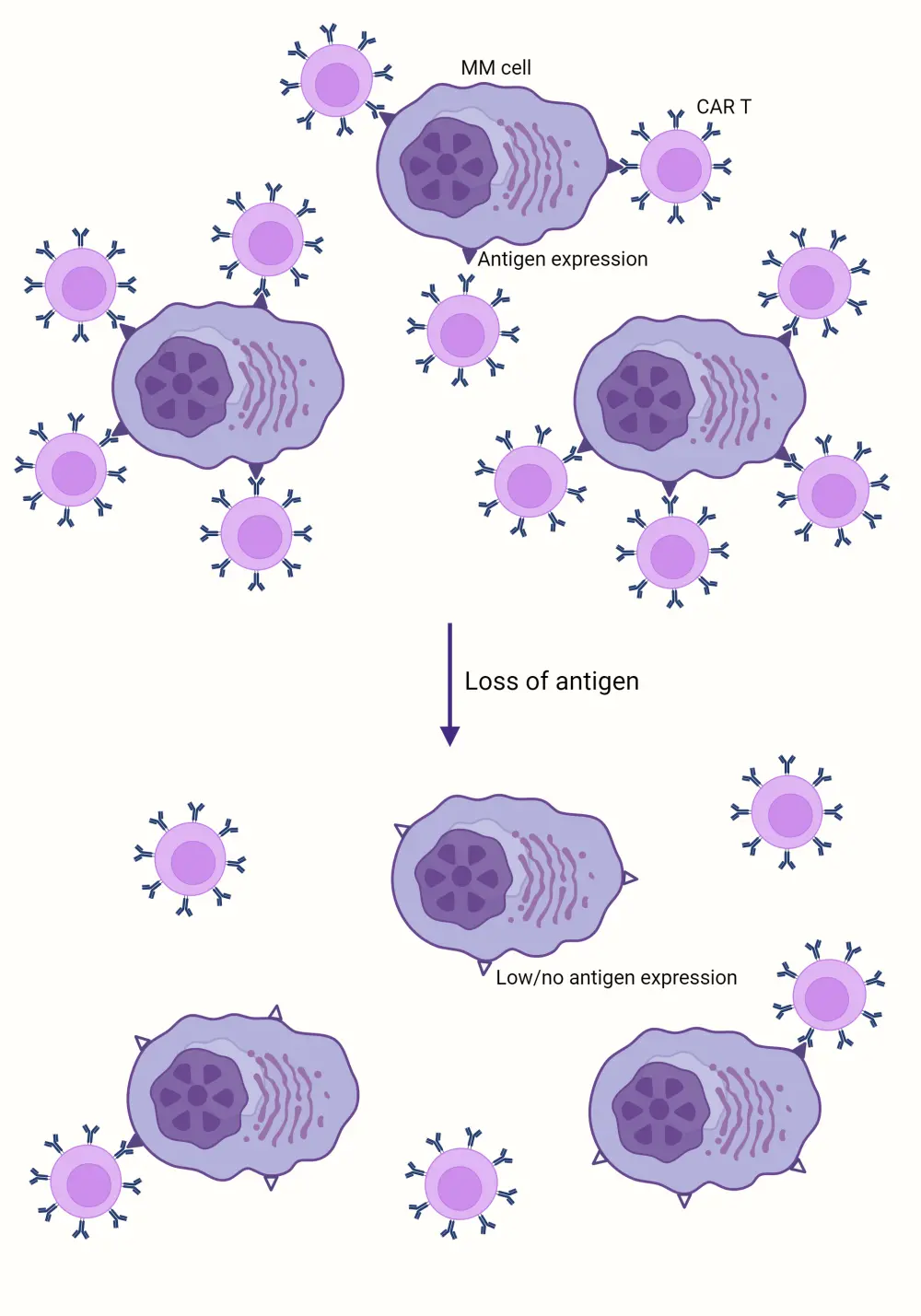
CAR, chimeric antigen receptor; MM, multiple myeloma.
Created with BioRender.com.
*Adapted from Golden, et al.3
|
Key learnings1 |
|---|
|
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?



