All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
The first-in-class peptide–drug conjugate, melphalan flufenamide (melflufen), is being widely investigated in the multiple myeloma (MM) setting in the hope to address the unmet clinical need of patients with heavily pretreated relapsed/refractory (R/R) MM (RRMM).1
The Multiple Myeloma Hub has previously reported data from a variety of trials evaluating melflufen for the treatment of MM. In particular, the phase II HORIZON study uncovered the encouraging clinical efficacy of melflufen in combination with dexamethasone (dex) for the treatment of patients with RRMM, and the combination is currently under priority review with a decision due in February 2021.2
Paul Richardson and colleagues recently published the latest data from the HORIZON study in the Journal of Clinical Oncology, in which melflufen plus dex exhibited an overall response rate (ORR) of 29%, median progression-free survival (PFS) of 4.2 months, and median overall survival (OS) of 11.6 months.1 For more information on the status of melflufen in combination with novel agents for the treatment of RRMM, listen to our podcast with Paul Richardson below. The promising safety and efficacy profiles of melflufen in combination with dex have provided the rationale for the further investigation of melflufen as part of a triplet regimen.
Melphalan flufenamide (melflufen) + novel agents for RRMM
At the 62nd American Society of Hematology (ASH) Annual Meeting and Exposition, Enrique Ocio presented the latest data from the phase I/IIa ANCHOR (OP-104; NCT03481556) study evaluating melflufen plus dex and either daratumumab (dara) or bortezomib (BTZ) for the treatment of patients with RRMM.3
Study design3
Patients were eligible for the study if they met the following criteria:
- RRMM
- aged ≥ 18 years
- received 1–4 prior lines of therapy
- refractory to, or intolerant of, an immunomodulatory drug (IMiD)
- patients assigned to the BTZ arm (n = 13) must not have been proteasome inhibitor (PI)-refractory
- patients assigned to the dara arm (n = 33) must not have received prior anti-CD38 monoclonal antibody therapy
The phase I dose escalation study followed a 3 × 3 design whereby patients received treatment as outlined in Figure 1.
Figure 1. Schema of phase I ANCHOR study for patients assigned to A, the BTZ arm, and B, the dara arm3
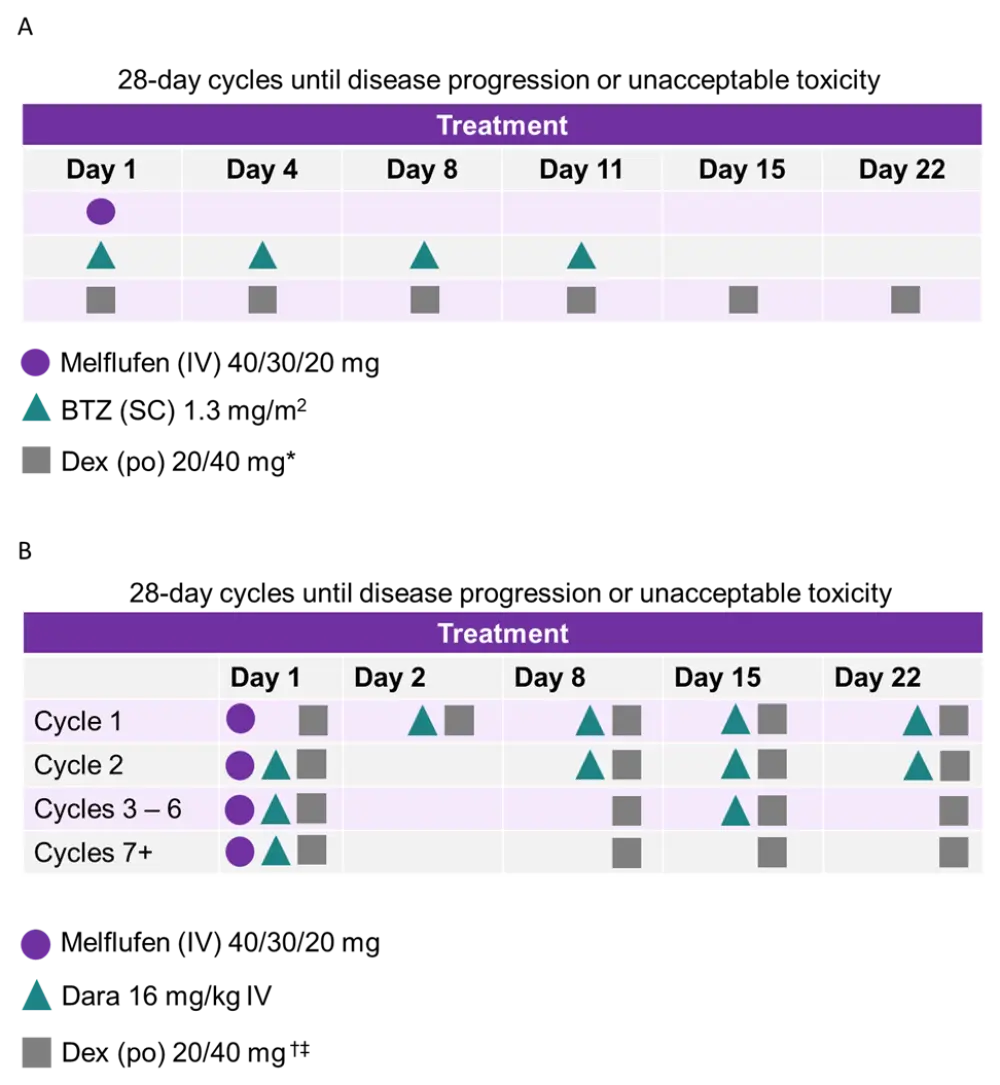
*,†Patients aged ≥ 75 years received 20 mg dex.
‡IV dex can substitute oral dex before dara infusion only.
In phase I, the recommended melflufen dose was based on any dose-limiting toxicities (DLTs) observed. When the optimal dose was found, a further 20 patients were recruited to each treatment arm, constituting the phase II of the study. The primary objectives from phases I and II were optimal melflufen dose in combination and ORR, respectively. The data cutoff was October 19, 2020.
Results3
Melflufen + dex + BTZ
- Patient characteristics are outlined in Table 1
- Treatment discontinuation (n = 5) occurred as a result of progressive disease (n = 2), adverse event (AE; n = 1), and other (n = 2)
- After a median follow-up of 12 months, PFS data were not mature
Table 1. Characteristics of patients in the BTZ arm of the ANCHOR trial3
|
Characteristic |
Melflufen + dex + BTZ (N = 13) |
|
BTZ, bortezomib; dex, dexamethasone; PI, proteasome inhibitor. |
|
|
Median age, years |
72 |
|
Median prior lines of therapy, n |
3 |
|
High-risk cytogenetics, % |
44 |
|
Refractory to last therapy, % |
77 |
|
Prior PI treatment, % |
92 |
Efficacy
- Patient responses to melflufen + dex in combination with BTZ at a median treatment duration of 8.7 months, are shown in Table 2. Eight patients (62%) are still on treatment.
Table 2. Patient responses in the BTZ arm of the ANCHOR trial3
|
Patient response |
Melflufen dose |
||
|
BTZ, bortezomib; CBR, clinical benefit rate; CR, complete response; ORR, overall response rate; PR, partial response; VGPR, very good partial response. |
|||
|
Best confirmed response, n |
30 mg (n = 6) |
40 mg (n = 7) |
Total (N = 13) |
|
> CR |
0 |
1 |
1 |
|
VGPR |
1 |
3 |
4 |
|
PR |
2 |
1 |
3 |
|
ORR, % |
50 |
71 |
62 |
|
CBR, % |
50 |
71 |
62 |
Safety
- Overall, no DLTs were reported
- The majority of Grade ≥ 3 treatment-related AEs (TRAEs) were hematological (Table 3)
- Serious TRAEs were observed in three patients which included pneumonia, neutropenia, thrombocytopenia, and syncope
- Death due to an AE, not considered linked to the study treatment, was observed in one patient
Table 3. Grade ≥ 3 TRAEs observed in patients in the BTZ arm of the ANCHOR trial3
|
Grade ≥ 3 TRAE, % |
Melflufen dose |
||
|
30 mg (n = 6) |
40 mg (n = 7) |
Total (N = 13) |
|
|
BTZ, bortezomib; TRAEs, treatment-related adverse events. |
|||
|
Any Grade ≥ 3 TRAEs |
83 |
100 |
92 |
|
Thrombocytopenia |
50 |
100 |
77 |
|
Neutropenia |
33 |
71 |
54 |
|
Anemia |
33 |
57 |
46 |
Melflufen + dex + dara
- There were no significant differences between the characteristics of patients in the melflufen dose groups (Table 4)
- The majority of patients had been exposed to alkylator therapy or transplant, but few were alkylator-refractory
- Of the patients who received 30 mg vs 40 mg melflufen:
- Median follow-up was 28.4 vs 16.9 months
- 33% vs 11% remained on treatment
- 67% vs 89% discontinued treatment due to
- progressive disease: 33% vs 44%
- AEs: 17% vs 26%
- other: 17% vs 19%
- The median number of treatment cycles was 19 vs 6, and the median treatment duration was 21.7 vs 6.2 months
- 33% vs 26% continued treatment with dara + dex after discontinuing melflufen
- Recommended phase II dose: 30 mg melflufen IV
Table 4. Characteristics of patients in the dara arm of the ANCHOR trial3
|
Characteristic |
Melflufen dose |
||
|
30 mg (n = 6) |
40 mg (n = 27) |
Total (N = 33) |
|
|
ASCT, allogeneic stem cell transplant; dara, daratumumab; ECOG, Eastern Cooperative Oncology Group; IMiD, immunomodulatory drug; ISS, International Scoring System; PI, proteasome inhibitor. *Determined by fluorescence in situ hybridization (FISH) and defined as t(4;14), t(14;16), t(14;20), del(17/17p), or gain(1q). |
|||
|
Median age, years |
57 |
66 |
63 |
|
Male sex, % |
50 |
70 |
67 |
|
Median prior lines of therapy, n |
2.5 |
2.0 |
2.0 |
|
High-risk cytogenetics, %* |
40 |
57 |
54 |
|
ECOG score 2, % |
17 |
7 |
9 |
|
ISS III, % |
0 |
11 |
9 |
|
Refractory to last therapy, % |
50 |
63 |
61 |
|
IMiD refractory, % |
50 |
67 |
64 |
|
PI refractory, % |
0 |
56 |
45 |
|
IMiD and PI refractory, % |
0 |
44 |
36 |
|
Alkylator refractory, % |
17 |
11 |
12 |
|
Prior ASCT / alkylator treatment, % |
83/83 |
78/89 |
79/88 |
Efficacy
- At the data cutoff, median duration of response was 12.6 months
- ORRs were comparable between patients receiving 30 mg vs 40 mg melflufen (Table 5)
- Median PFS was 12.9 months (95% CI, 7.7‒15.4)
- OS data was immature at 18.4 months of follow-up
Table 5. Patient responses in the dara arm of the ANCHOR trial3
|
Patient response |
Melflufen dose |
||
|
CBR, clinical benefit rate; CR, complete response; dara, daratumumab; ORR, overall response rate; PR, partial response; VGPR, very good partial response. |
|||
|
Best confirmed response, n |
30 mg (n = 6) |
40 mg (n = 27) |
Total (N = 33) |
|
> CR |
0 |
2 |
2 |
|
VGPR |
4 |
6 |
10 |
|
PR |
1 |
11 |
12 |
|
ORR, % |
83 |
70 |
73 |
|
CBR, % |
83 |
74 |
76 |
Safety
- Overall, no DLTs were reported
- The majority of Grade ≥ 3 TRAEs were hematological (Table 6)
- Serious AEs were observed in 45% of patients (67% vs 41% of patients in the 30 mg vs 40 mg cohorts) which included pneumonia, febrile neutropenia, influenza, parainfluenza, sepsis, and urinary tract infection
- Death due to AEs was observed in four patients, one of which occurred in the 40 mg cohort (sepsis), and was possibly melflufen-related
- The most common AEs resulting in dose reduction were thrombocytopenia and neutropenia
- The most common AEs resulting in discontinuation of any study drug were thrombocytopenia, neutropenia, sepsis, and anemia
Table 6. Grade ≥ 3 TRAEs observed in patients in the dara arm of the ANCHOR trial3
|
Grade ≥ 3 TRAE, % |
Melflufen dose |
||
|
30 mg (n = 6) |
40 mg (n = 27) |
Total (N = 33) |
|
|
Dara, daratumumab; TRAEs, treatment-related adverse events. |
|||
|
Overall |
83 |
89 |
88 |
|
Thrombocytopenia |
50 |
78 |
73 |
|
Neutropenia |
83 |
63 |
67 |
|
Anemia |
50 |
19 |
24 |
|
Lymphopenia |
0 |
7 |
6 |
|
Febrile neutropenia |
17 |
4 |
6 |
|
Pneumonia |
0 |
7 |
6 |
Conclusions
Melflufen in combination with dex and BTZ/dara demonstrated promising clinical activity in patients with RRMM, with ORRs of 73% and 62% in the dara and BTZ cohorts, respectively. Safety profiles of the melflufen-containing triplets were similar to that of melflufen + dexa alone, and the triplet regimens were well tolerated in patients with RRMM. Hematological TRAEs were controlled with dose reductions and no DLTs were observed across the entire study.
A dose of 30 mg melflufen has been recommended in patients receiving it in combination with dex and dara, and in light of these data, a phase III trial evaluating melflufen + dex + dara vs dara single-agent has started recently recruiting patients with RRMM (LIGHTHOUSE study, NCT04649060).
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?


 Paul Richardson
Paul Richardson