All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
What are the main discrepancies between MM treating centers in Brazil?
Featured:
Over the last 20 years, there have been significant advances in multiple myeloma (MM) treatment, yet there remain significant inequalities in access to treatment and survival.1 Patient outcomes in clinical trials do not necessarily reflect the real-world effectiveness of these interventions.1
The MMyBRave trial was set with the aim to establish the clinical and demographic characteristics, as well as care pathways and outcomes for patients with MM in Brazil.1 The Multiple Myeloma Hub was pleased to speak to Vania Tietsche de Moraes Hungria, Clínica São Germano, São Paolo, BR, about her takeaways on the findings of this study, which are summarized below.
What are the main discrepancies between MM treating centers in Brazil?
Study design1
A total of 943 patients were recruited from 17 institutions with a diagnosis of MM based on the latest International Myeloma Working Group (IMWG) criteria. The MMyBRave trial reported the patient outcomes, including overall survival (OS) and predictors of OS. The analysis presented at the 64th American Society of Hematology (ASH) Annual Meeting and Exposition focused on the comparison of OS by baseline characteristics (Table 1), eligibility for transplant, and whether treatment was received in private or public institutions. Of note, a higher proportion of patients treated in public institutions presented with Durie-Salmon Stage III and International Staging System Stage III than in private institutions.
Table 1. Baseline characteristics by type of center*
|
ISS, International Staging System; IQR, interquartile range. |
|||
|
Characteristic, % (unless otherwise stated) |
Public |
Private |
p value |
|---|---|---|---|
|
Median age (IQR), years |
68 (60–76.8) |
69 (61–78) |
0.273 |
|
Sex |
|
|
|
|
Female |
48.6 |
(43.6 |
0.149 |
|
Male |
51.4 |
56.4 |
|
|
Race |
|
|
|
|
White |
61.8 |
60.1 |
0.640 |
|
Other or unknown |
38.2 |
39.9 |
|
|
Durie-Salmon stage |
|
|
|
|
I |
5.4 |
8.1 |
0.001 |
|
II |
16.2 |
15.8 |
|
|
III |
(66.9) |
56.8 |
|
|
Not performed/unavailable |
11.5 |
19.3 |
|
|
ISS stage |
|
|
|
|
I |
13.3 |
26.7 |
<0.001 |
|
II |
20.2 |
22.6 |
|
|
III |
27.4 |
25.7 |
|
|
Not performed/unavailable |
39.1 |
25.0 |
|
Results1
OS was associated with multiple factors, including center type (Figure 1), age, and Durie-Salmon stage. Each of these factors resulted in a significant difference in OS. The type of center (public or private) was identified as an important factor in disparity despite the fact the number of patients undergoing transplant or eligible for transplant was not significantly different between centers. Transplant eligibility did independently have an impact on median OS, with patients who are transplant-eligible having a median OS of 93 months vs 49 months in the transplant-ineligible group. This difference was maintained regardless of the type of center these patients attended.
Figure 1. Median overall survival by center type*
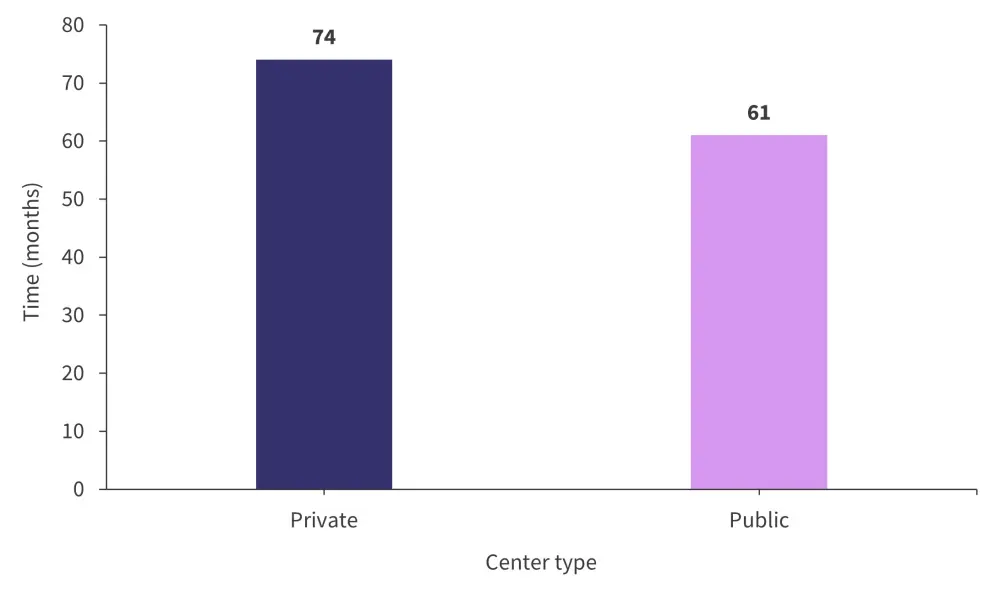
*Adapted from Hungria.1
In addition to OS, there was also a difference in the frontline treatment patients received depending on whether they were treated in a public or private institution. Patients treated in private institutions were more likely to receive bortezomib as a frontline therapy compared with primarily thalidomide and melphalan-based treatments in public centers.
Conclusion
The treatment of MM in Brazil is improving over time, but disparities in therapeutic options and patient outcomes still exist. The MMyBRave trial identified the type of treating center (private or public) as one source of disparity. The cause of difference in outcomes across centers is not yet fully understood; however, access to novel therapies may be a key factor.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?


 Vania Tietsche de Moraes Hungria
Vania Tietsche de Moraes Hungria