All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
VTE prophylaxis: Real-world practices and IFM clinical guidelines
Venous thromboembolic events (VTE) are commonly associated with multiple myeloma (MM) and can have detrimental effects such as treatment discontinuation, increased bleeding, and long-term complications that all negatively impact prognosis. The cause of VTE in MM is not fully understood; however, an association has been observed between immunomodulatory agent (IMiD)-based therapies in combination with dexamethasone, as well as inappropriate use of thromboprophylaxis.1
To more investigate the causative contributions of these factors, the Intergroupe Francophone du Myélome (IFM) conducted a large survey for clinicians regarding their current thromboprophylaxis practices, and used this to develop guideline recommendations for thromboprophylaxis of patients with MM.
The Multiple Myeloma Hub is pleased to summarize the results of this survey and the IFM clinical guidelines below.
Results of the survey1
- In total, 84 clinicians were surveyed across France and Belgium with the majority (83.3%) being hospital practitioners from a variety of practice settings. Overall, 98.8% of responders considered thromboprophylaxis important in patient care
- Most clinicians (70.9%) opted to continue with thromboprophylaxis until the end of IMiD-based therapy in both first line and relapsed/refractory settings
- Two thirds of participants also indicated that they would still prescribe thromboprophylaxis when a non IMiD-based treatment was chosen
The considerations clinicians made for practice before initiating thromboprophylaxis are outlines in Figure 1.
Figure 1. Survey results on the considerations before initiating thromboprophylaxis*

IMiD, immunomodulatory agent; IMWG, International Myeloma Working Group; VTE, venous thromboembolic event.
*Data from Frenzel, et al.1
Antithrombotic drugs dosing and administration1
- The preferred antithrombotic thromboprophylaxis was direct oral anticoagulants (DOAC), followed by low-molecular-weight heparin (LMWH), and then aspirin
- Overall, 13% had never considered DOAC thromboprophylaxis
- Overall, 15% stated they never prescribe aspirin but among those who did, 75 mg daily dose was preferred
- The majority of patients did not receive any additional anticoagulation therapy, and when given it was preferentially given under cardiac supervision
- Two thirds of clinicians prescribed enoxaparin 0.4 mL vs one third who prescribed <175 IU/Kg tinzaparin daily
- In total, 78% preferred prescribing 2.5 mg apixaban twice a day vs 18% who preferred 10 mg rivaroxaban daily
The justifications for not prescribing each drug class include:
- LMWH; administered via subcutaneous route leading to difficulties in patients after extended treatment, as well as their use in patients with severe renal failure.
- DOACs; concerns regarding lack of approval in MM, risk of bleeding, absence of antidote, and renal impairment.
IFM clinical guidance1
With the aim to improve thromboprophylaxis in patients with MM, the IFM developed an algorithm to aid in clinical decision-making. This algorithm is presented in Figure 2.
Figure 2. IFM clinical recommendations for thromboprophylaxis initiation*
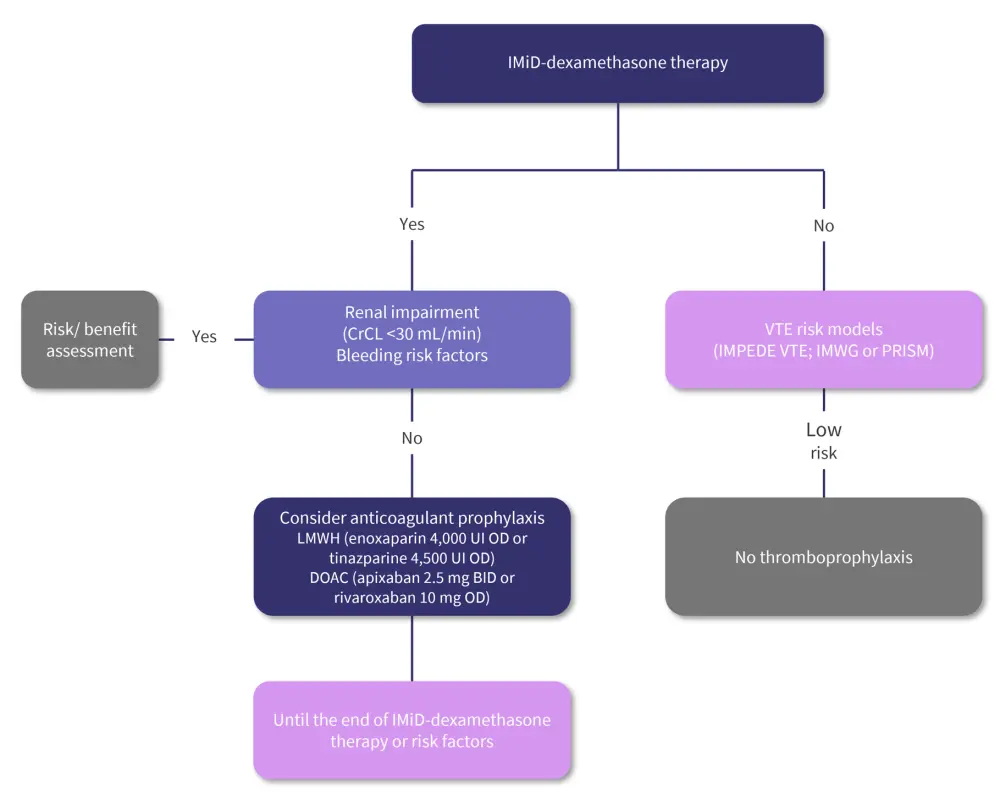
BID, twice daily; CrCL, creatinine clearance; DOAC, direct oral anticoagulant; IMiD, immunomodulatory agent; IMWG, International Myeloma Working Group; IU, international unit; LMWH, low-molecular-weight heparin; OD, once daily; PRISM, Precision Intervention Smoldering Myeloma; VTE, venous thromboembolism.
*Adapted from Frenzel, et al.1
The choice of an IMiD-dexamethasone-based therapy emerged as the key factor to initiating the decision algorithm, based on published data showing that the risk of VTE is up to 25% higher in those being treated with IMiDs, particularly in combination with dexamethasone. Alongside existing therapy, there are several highlighted risk factors including renal impairment and bleeding risks, which are also associated with a higher likelihood of VTEs.
Of note in the IFM report, aspirin is described as an inappropriate thromboprophylactic agent that should not be considered. This is due to trial data, such as that in the MELISSE study which found that VTEs were observed in 7% of patients treated with aspirin vs 3% of patients treated with LMWH. Furthermore, in an additional trial, 1.7% of patients treated with aspirin were diagnosed with a pulmonary embolism compared with zero in the LMWH group.
Conclusions
There are inconsistencies in current real-world treatment practices for VTE prophylaxis, highlighting a need for better understanding and clearer clinical guidelines. The IFM highlights that the consideration of VTE prophylaxis is vital for all patients being treated with IMiDs in combination with dexamethasone and other key risk factors including renal failure or bleeding risk.
This novel algorithm can help guide treatment decisions. However, it is important to note that in-depth clinical trials on the implementation of this guidance are lacking, and to further improve treatment guidelines and patient outcomes, long-term follow-up, and a better understanding of VTEs in MM is required.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?

