All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Teclistamab, a BCMA/CD3 bispecific antibody for patients with RRMM: Phase I MajesTEC-1 trial results
Multiple myeloma remains an incurable disease despite recent advances in treatment, and so patients may undergo multiple rounds of remission and relapse. Each relapse results in poorer outcomes; therefore, there is a considerable unmet medical need for patients who are refractory to proteasome inhibitors, immunomodulatory drugs, and/or anti-CD38 monoclonal antibodies. Agents with new modes of action, including B-cell maturation antigen (BCMA)-targeting immunotherapies, offer promising treatment options for patients with relapsed and refractory multiple myeloma (RRMM).
Teclistamab is a bispecific antibody with a CD3 arm that binds to T cells and a BCMA arm that binds to myeloma cells. The redirected T cells then subsequently kill the myeloma cells by releasing perforins and granzymes. Teclistamab is currently being tested in the phase I MajesTEC-1 trial (NCT03145181) for patients with RRMM. The results of this trial led the U.S. Food and Drug Administration (FDA) to grant teclistamab Priority Medicine (PRIME) designation earlier in the year and breakthrough therapy designation on June 1, 2021.1 The results of this trial were recently presented at the 2021 American Society of Oncology (ASCO) Annual Meeting by Amrita Krishnan2 and the European Hematology Association (EHA)2021 Virtual Congress by Neils van de Donk3. The results were also recently published in The Lancet by Saad Usmani,4 and are summarized below.
Study design and patient characteristics2,3
- A two-part, multicenter (12 centers across the US, France, the Netherlands, Spain, and Sweden), phase I study to investigate the safety and efficacy of teclistamab in patients with RRMM (Figure 1).
- Part 1: A dose escalation to find the recommended phase II dose (RP2D).
- Part 2: A dose expansion to determine the safety and tolerability at this RP2D dose and to measure the anti-tumor activity, pharmacokinetics, and pharmacodynamics.
Figure 1. Study design*
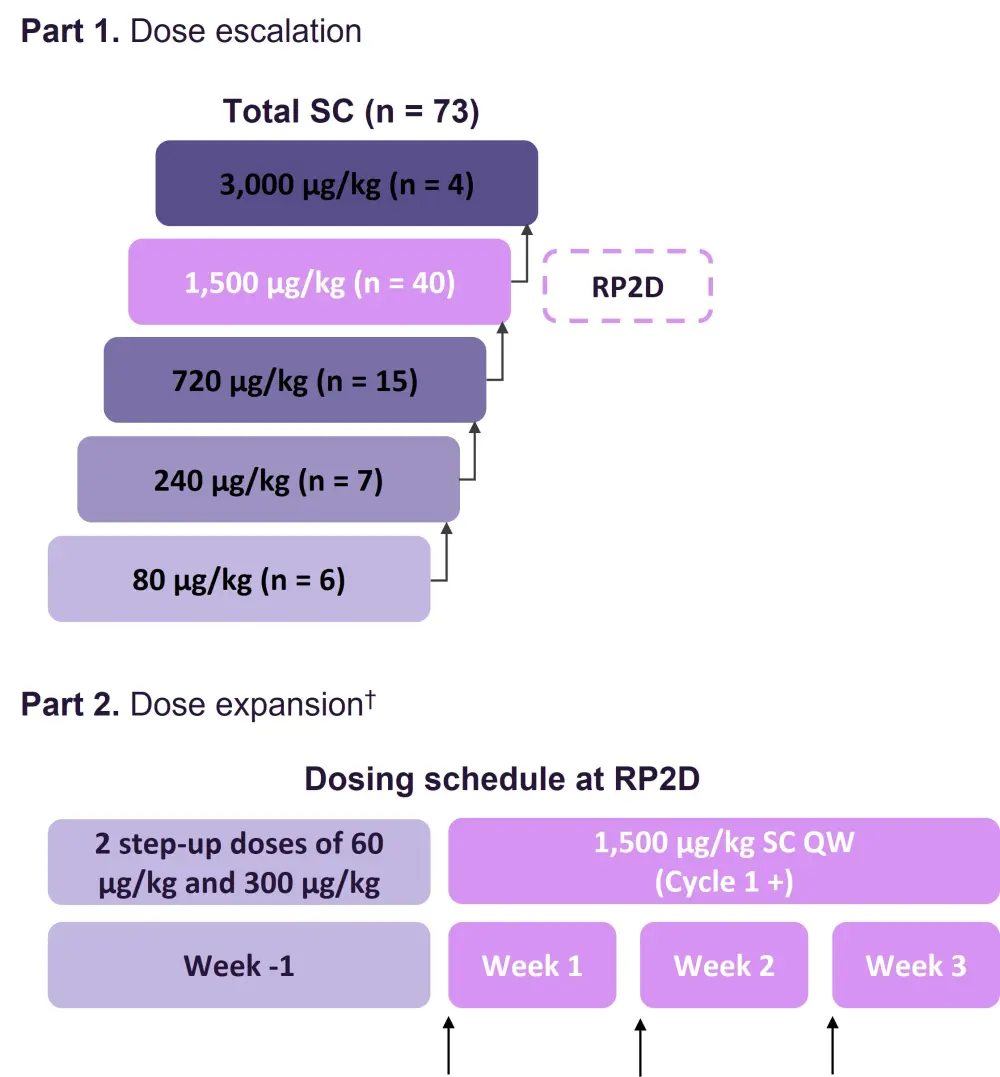
QW, once weekly; RP2D, recommended phase II dose; SC, subcutaneous.
*Adapted from Krishnan, et al.2
†Arrows represent SC administration of teclistamab.
- The study was initially started using both intravenous (IV) and subcutaneous (SC) dosing.
- Overall, 157 patients with RRMM were enrolled at the time of data cut-off (March 29, 2021).
- n = 84 were treated with the IV formulation.
- n = 73 were treated with the SC formulation, of which 40 were treated at the RP2D.
- RP2D = 1,500 µg/kg SC once weekly (based on safety, efficacy, PK, and pharmacodynamic data).
- Step-up dosing was used to mitigate the risk of severe cytokine release syndrome (CRS).
- The use of steroid premedication was limited to the step-up and first full dose of teclistamab.
- The maximum tolerated dose was not reached.
- Baseline characteristics can be seen in Table 1 for the total SC cohort and the RP2D cohort.
- Patients were similar across the cohorts.
- Patients were heavily pretreated, with a median of five prior lines of therapy.
- Most patients (79%) were triple-class refractory and refractory to the last line of therapy.
- Over a third (38%) of the patients were penta-drug refractory.
Table 1. Baseline characteristics*
|
IMiD, immunomodulatory drug; ISS, International Staging System; mAb, monoclonal antibody; PI, proteosome inhibitor; RP2D, recommended phase II dose; SC, subcutaneous. |
||
|
Characteristic |
Total SC |
RP2D |
|---|---|---|
|
Median age (range), years |
64.0 (39–84) |
62.5 (39–84) |
|
Male (%) |
59 |
65 |
|
Median time since diagnosis, year (range) |
5.9 (0.8–23.5) |
5.7 (0.8–17.4) |
|
Extramedullary soft tissue plasmacytomas ≥1, (%) |
15 |
20 |
|
Bone marrow plasma cells ≥60% (%) |
18 |
8 |
|
High-risk cytogenetics (%) |
30 |
37 |
|
ISS stage (%) |
|
|
|
I |
50 |
62 |
|
II |
35 |
28 |
|
II |
15 |
10 |
|
Median prior line of therapy (range) |
5 (2–14) |
5 (2–11) |
|
Prior transplantation (%) |
86 |
85 |
|
Refractory status (%) |
|
|
|
PI |
89 |
88 |
|
Carfilzomib |
67 |
68 |
|
IMiD |
96 |
95 |
|
Pomalidomide |
75 |
70 |
|
Anti-CD38 mAb |
93 |
98 |
|
Triple class |
79 |
83 |
|
Penta drug |
38 |
38 |
|
Refractory to last line of therapy |
88 |
83 |
Results2,3
Safety
- The safety profile of teclistamab can be seen in Table 2.
- Teclistamab was well tolerated, with the most common hematologic adverse event (AE) being neutropenia.
- In the subset of patients who experienced Grade 3 or 4 cytopenia, the onset was generally confined to the step-up dosing in Cycles 1 and 2.
- The most common non-hematologic AEs were primarily of Grade 1 or 2 and included CRS, followed by nausea and fatigue.
Table 2. AEs occurring in ≥20% of patients in the SC cohort*
|
AE, adverse event; CRS, cytokine release syndrome; RP2D, recommended phase II dose; SC, subcutaneous. |
||||
|
|
Total SC |
RP2D |
||
|---|---|---|---|---|
|
AE* |
Any Grade |
Grade 3/4 |
Any Grade |
Grade 3/4 |
|
Hematologic (%) |
|
|
|
|
|
Neutropenia |
63 |
44 |
65 |
40 |
|
Anemia |
51 |
26 |
50 |
28 |
|
Thrombocytopenia |
41 |
21 |
45 |
20 |
|
Leukopenia |
26 |
12 |
33 |
18 |
|
Nonhematologic (%) |
|
|
|
|
|
CRS |
60 |
0 |
70 |
0 |
|
Nausea |
32 |
0 |
33 |
0 |
|
Fatigue |
29 |
1 |
38 |
3 |
|
Injection site erythema |
27 |
0 |
33 |
0 |
|
Headache |
25 |
0 |
20 |
0 |
|
Diarrhea |
23 |
3 |
23 |
5 |
|
Cough |
21 |
1 |
10 |
0 |
|
Pyrexia |
21 |
0 |
13 |
0 |
- Infections were reported in 51% of the SC cohort, of which 21% were Grade 3/4.
- Grade 1 neurotoxicity occurred in one patient treated at the RP2D (the patient remained on treatment and the event resolved without any intervention).
- In the SC cohort, 42% of patients experienced injection site reactions that were mild and easily manageable.
- Two deaths occurred due to AEs (general health deterioration and sepsis) in the SC cohort; neither were related to teclistamab and neither occurred at the RP2D.
- CRS events were either Grade 1/2 and were generally confined to the step-up and first full doses.
- The median time to CRS onset as well as the median duration of CRS in both cohorts was 2 days.
- Supportive measures included the use of tocilizumab, steroids, and low flow oxygen by nasal cannula in 60%, 22%, 20%, and 5% of patients, respectively, in the SC cohort.
- CRS resolved in all patients.
Efficacy
- As of the data cut-off, the median duration of follow-up for the 40 patients treated at the RP2D was 6.1 months (range, 1.2–12.2 months).
- The overall response rate at the RP2D dose was 65% (Figure 2).
- 58% of patients achieved a ≥ very good partial response.
- 40% of patients achieved a ≥ complete response (CR).
Figure 2. Overall response rate*
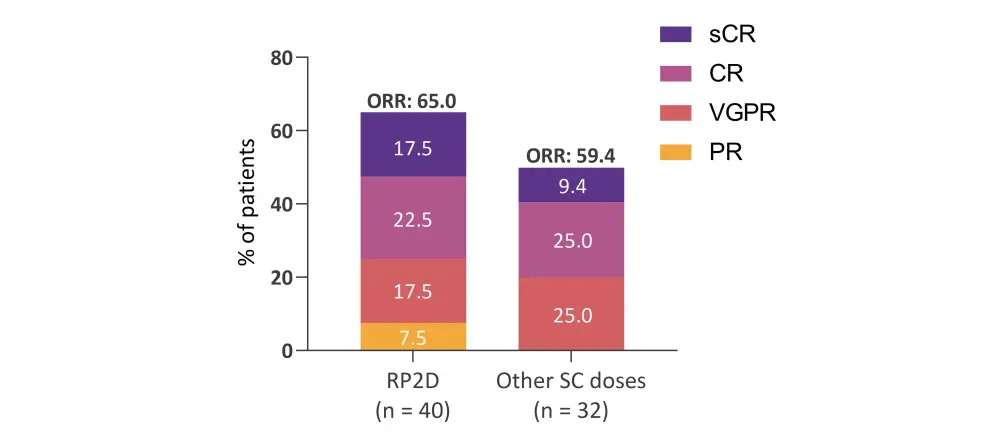
CR, complete response; ORR, overall response rate; PR, partial response; RP2D, recommended phase II dose; SC, subcutaneous; sCR, stringent complete response; VGPR, very good partial response.
*Adapted from Krishnan, et al.2
- Of note, in the 33 patients with triple-class refractory disease who were treated at the RP2D, the overall response rate was 61%.
- All six evaluable patients in the RP2D cohorts achieved measurable residual disease (MRD)-negative CR or stringent CR at a sensitivity of 10−5 (n = 5) or 10−6 (n = 1).
- Responses were durable and deepened over time.
- The median duration of response at the RP2D was not reached.
- 85% of responders treated at the RP2D were alive and continuing treatment after median follow-up of 7.1 months.
- 80% of patients in the total SC cohort were alive and continuing treatment after median follow-up of 9.3 months.
Pharmacokinetics and pharmacodynamics
- Teclistamab exposure was sustained across the dosing interval at the RP2D. Importantly, the mean serum levels of teclistamab before the next dose administration was above the target exposure level (based on the EC50 from an ex vivo cytotoxicity assay).
- Immunogenicity was low and did not impact the pharmacokinetics.
- Only one patient treated with SC teclistamab had low titer anti-drug antibodies, and this patient was not treated at the RP2D.
- After weekly SC dosing, PD-1 positive T cells were induced in the periphery, with consistent T cell activation observed at the RP2D.
Conclusion
Teclistamab showed encouraging efficacy and safety in patients with triple-class RRMM, including high-risk patients with extramedullary disease. These results support the RP2D (1,500 µg/kg SC once weekly) of teclistamab and demonstrate it to be well tolerated, with no new safety signals identified. CRS events with the step-up dosing plan were low-grade and manageable. The response rate to teclistamab was high, with durable responses that deepened over time. With the SC dosing, drug levels exceeded target exposure, and T cell induction was observed. The open-label phase II expansion study (NCT04557098) of teclistamab at the RP2D is currently underway. Future studies will evaluate teclistamab in earlier lines of therapy as well as in combination with other agents, such as immunomodulatory drugs and anti-CD38 antibodies.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?

