All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Results from the LUMMICAR-2 study of CT053, a human BCMA-specific CAR-T therapy for patients with RRMM
Featured:
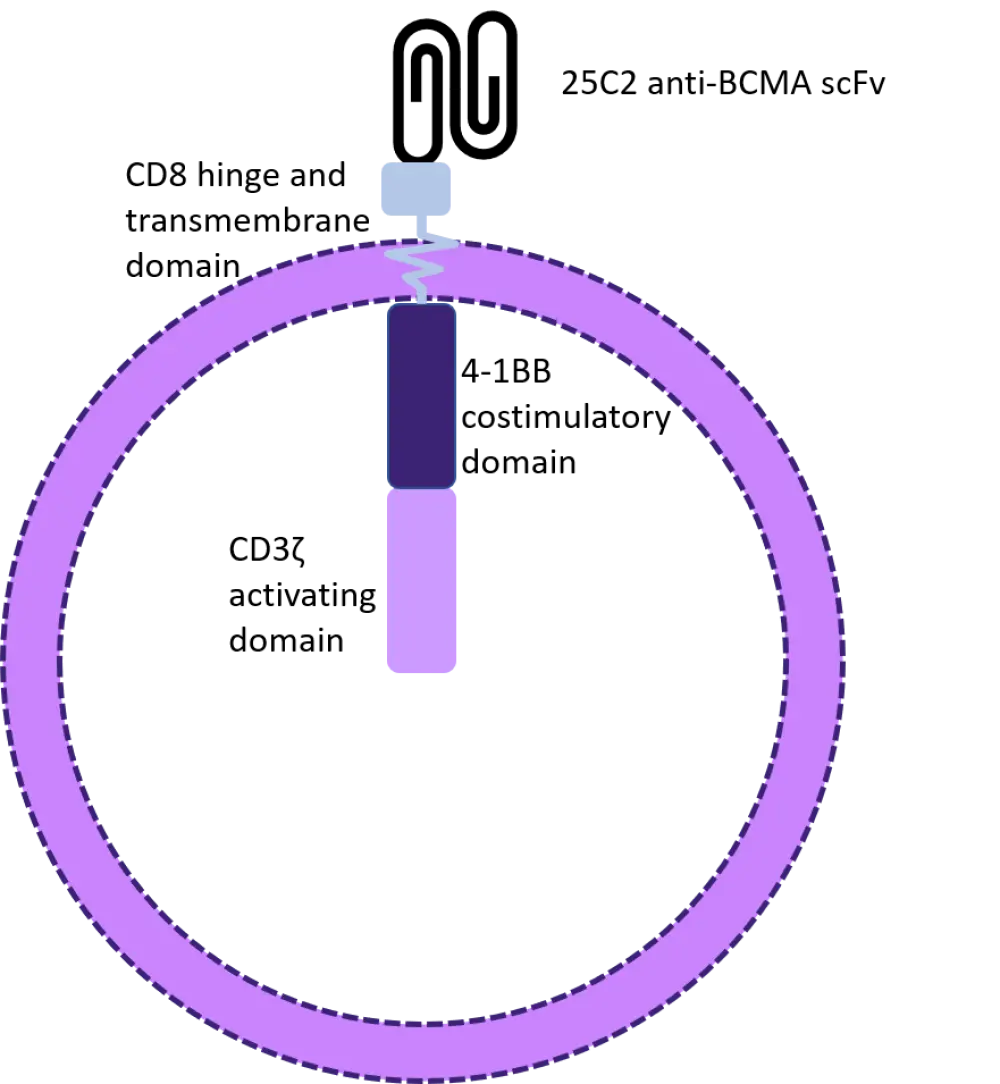
CT053 is an investigational, second-generation chimeric antigen receptor (CAR) T-cell product with a human B-cell maturation antigen (BCMA)-specific single-chain fragment variant (25C2) and high binding affinity; see Figure 1. The U.S. Food and Drug Administration (FDA) granted CT053 regenerative medicine advanced therapy designation in 2019. The advantage of this CAR T-cell therapy is that the manufacturing time is shorter than the average CAR T-cell production, at only 8−10 days between apheresis and infusion.1 For patients with relapsed/refractory multiple myeloma (RRMM), a reduced wait time before CAR-T infusion can have a significant impact on survival outcome.
Figure 1. CT053 structure2
During the 62nd American Society of Hematology (ASH) Annual Meeting and Exposition, several presentations discussed the results of phase I and phase Ib/II trials investigating CT053 in patients with RRMM.
CT053 was first evaluated in a phase I investigator-initiated study in China, which enrolled 24 patients with a median of 4.5 prior treatment lines.1 Nine patients are still in complete response (CR) or stringent CR 24 months after infusion. Overall, the authors reported a median duration of response of 21.8 months, with progression-free survival rates at 6 and 12 months of 87% and 60.9%, respectively.
The results from LUMMICAR-1 (NCT03975907), another phase I study conducted in China, were presented at ASH by Wenming Chen,3 while Shaji Kumar presented the results of the ongoing LUMMICAR-2 (NCT03915184) trial, conducted in North America.2 This article will summarize the latter.
Study design2
Patients were eligible for the LUMMICAR-2 trial if they had a diagnosis of RRMM, had previously received ≥ 3 lines of treatment, including a proteasome inhibitor and an immunomodulatory drug, and were refractory to the last line of therapy. The trial design is shown in Figure 2.
The key objectives of the phase Ib portion were to evaluate the safety of CT053 and find the maximum tolerated dose for the phase II trial. The efficacy of CT053 would be assessed in the phase II stage in terms of overall response rate.
Figure 2. Trial schedule2

Cy, cytarabine; D, day; Flu, fludarabine; M, month.
In the phase Ib study, 20 patients were enrolled. Of these, 25% presented extramedullary disease at baseline, 55% had high-risk cytogenetics, and 50% were classed as penta-refractory.
Key points2
In terms of safety, CT053 exhibited a profile similar to other CAR T-cell treatments, with cytopenia being the most common adverse event experienced. Cytokine release syndrome (CRS) occurred in 77% of patients on dose level 0 (DL0; 1.5−1.8 × 108 cells; n = 14) and 83% of those on dose level 1 (DL1; 2.5−3.0 × 108 cells; n = 6). For both groups, around 50% of patients experienced Grade 1 CRS. There were no cases of Grade ≥ 3 CRS recorded.
In both DL0 and DL1, CT053 rapidly depleted plasma cells in the bone marrow of patients. A deep reduction in M-protein was seen within a month following CT053 infusion, along with a decrease in both the serum-free light chain and soluble BCMA.
CT053 CAR T cells were detectable 3−7 days after infusion and reached a peak at 7−14 days. The median peak did not differ significantly between DL0 and DL1. To date, CAR T cells were still detectable after 6 months from infusion.
An overall response rate of 94% was seen in the 18 patients who had at least 8 weeks of follow-up data (stringent CR/CR, n = 5; very good partial response, n = 5; partial response, n = 7). The depth of response appeared to improve with longer follow-up.
Conclusion
The outcome of treatment is time-sensitive for patients with RRMM. CT053, with its reduced manufacturing time, represents a key advantage over other available CAR T-cell products. In line with other BCMA-directed CAR T-cell therapies, CT053 demonstrated an acceptable safety profile and promising efficacy in this heavily pre-treated population.
How is CT053 different from other BCMA-directed CAR T-cell therapies?
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?


 Shaji Kumar
Shaji Kumar