All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Response to anti-BCMA CAR T therapy in patients with renal dysfunction
Up to half of patients with multiple myeloma (MM) experience renal impairment. While the exact underlying pathology may vary, myeloma cast nephropathy is the most common form of kidney damage seen in MM. Damage to the kidneys is associated with a poorer prognosis and reduced overall survival (OS).
Anti B-cell maturation antigen chimeric antigen receptor (CAR) T-cell therapy has shown great success in treating patients, however the impact of this treatment on renal impairment is unknown. In a recent article by He, et al., the impact of CAR T-cell therapy in relapsed/refractory (RR) patients with MM who had impaired renal function (IRF) was assessed.1
Study design
In total, 59 patients with RRMM were enrolled onto this study. Patients were split into two groups according to renal function assessed by estimated glomerular filtration rate (eGFR). The IRF group had an eGFR <90 mL/min/1.73 m2, whereas the normal renal function (NRF) group had an eGFR ≥90 mL/min/1.73 m2. CAR T cells were cultured for a median of 16 days (range, 12–25 days) prior to infusion. The dosing schedule is shown in Figure 1.
Figure 1. Study design*
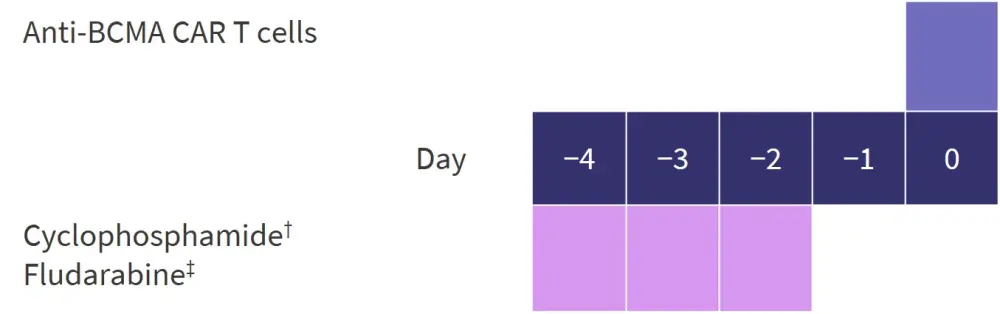
BCMA, B-cell maturation antigen; CAR, chimeric antigen receptor.
*Adapted from He, et al.1
†20 mg/kg daily.
‡25 mg/m2 of body surface area daily.
The median dose of murine CAR T cells administered was 10.5 × 106/kg (4.93–25.00 × 106/kg), whereas the median fully human CAR T-cell dose was 1.00 × 106/kg (0.50–6.00 × 106/kg).
Results
Patient characteristics
The mean age of enrolled patients was 55 years (range, 34−70 years) and 40.7% were female. Patients had undergone a median of 4 previous lines of therapy (range, 3−11 lines of therapy). Over 90% of the patients had a Durie-Salmon stage of III, so had advanced RRMM. High-risk cytogenetic features were increased in the IRF group at 94.4% compared with 68.3% in the NRF group (Table 1).
Table 1. Patient baseline characteristics*
|
CAR, chimeric antigen receptor; eGFR, estimated glomerular filtration rate; IRF, impaired renal function; ISS, international staging system; NRF, normal renal function. |
||||
|
Characteristics |
IRF (n = 18) |
NRF (n = 41) |
All patients |
|
|---|---|---|---|---|
|
Median age (range), years |
59 (44–70) |
53 (34–69) |
55 (34–70) |
|
|
Sex, % |
Male |
55.6 |
61.0 |
59.3 |
|
|
Female |
44.4 |
39.0 |
40.7 |
|
Durie-Salmon stage, % |
I |
0 |
2.4 |
1.7 |
|
|
II |
0 |
4.9 |
3.4 |
|
|
III |
100 |
92.7 |
94.9 |
|
ISS stage, % |
I |
27.8 |
46.4 |
40.7 |
|
|
II |
27.8 |
39.0 |
35.6 |
|
|
III |
44.4 |
14.6 |
23.7 |
|
Myeloma type, % |
IgG-Kappa |
27.8 |
17.1 |
20.3 |
|
|
IgG-Lambda |
16.7 |
36.6 |
30.5 |
|
|
IgA-Kappa |
0 |
12.2 |
8.5 |
|
|
IgA-Lambda |
5.5 |
2.4 |
3.4 |
|
|
IgD-Lambda |
16.7 |
2.4 |
6.8 |
|
|
Light chain Kappa |
5.5 |
12.2 |
10.2 |
|
|
Light chain-Lambda |
27.8 |
9.8 |
15.2 |
|
|
Non-secretor |
0 |
7.3 |
5.1 |
|
High risk cytogenetics, % |
94.4 |
68.3 |
76.3 |
|
|
Median urea (range), mmol/L |
4.92 (2.45−23.30) |
3.70 (1.40−8.50) |
1.69 (1.40−23.30) |
|
|
Median creatinine (range), µmol/L |
92 (64−410) |
64 (29−89) |
72 (29−410) |
|
|
Median eGFR (range), mL/min/1.73m2 |
72.0 (13.9−89.3) |
101.4 (91.0−132.6) |
96.5 (13.9−132.6) |
|
|
Median time from diagnosis (range), years |
3.0 (0.7−9.3) |
3.3 (0.7−12.6) |
3.1 (0.7−12.6) |
|
|
Median lines of prior treatment (range), n |
4 (3−10) |
4 (3−11) |
4 (3−11) |
|
|
CAR T structure, % |
Murine |
83.3 |
53.7 |
62.7 |
|
|
Fully human |
16.7 |
47.3 |
37.3 |
|
Median CAR T-cell dosage (range), 106/kg |
|
10.1 (0.5−21.0) |
6.0 (0.5−25.0) |
7.5 (0.5−25.0) |
|
|
Murine |
10.29 (4.93−21.00) |
11.35 (5.00−25.00) |
10.50 (4.93−25.00) |
|
|
Fully human |
0.71 (0.50−1.00) |
1.00 (0.50−6.00) |
1.00 (0.50−6.00) |
Efficacy
The probability of achieving remission was not significantly different between the two renal groups and the overall response rate is shown in Figure 2. Disease progression occurred in 83.3% of patients in the IRF group and 88.9% of patients died during follow-up.
Figure 2. Response rates*
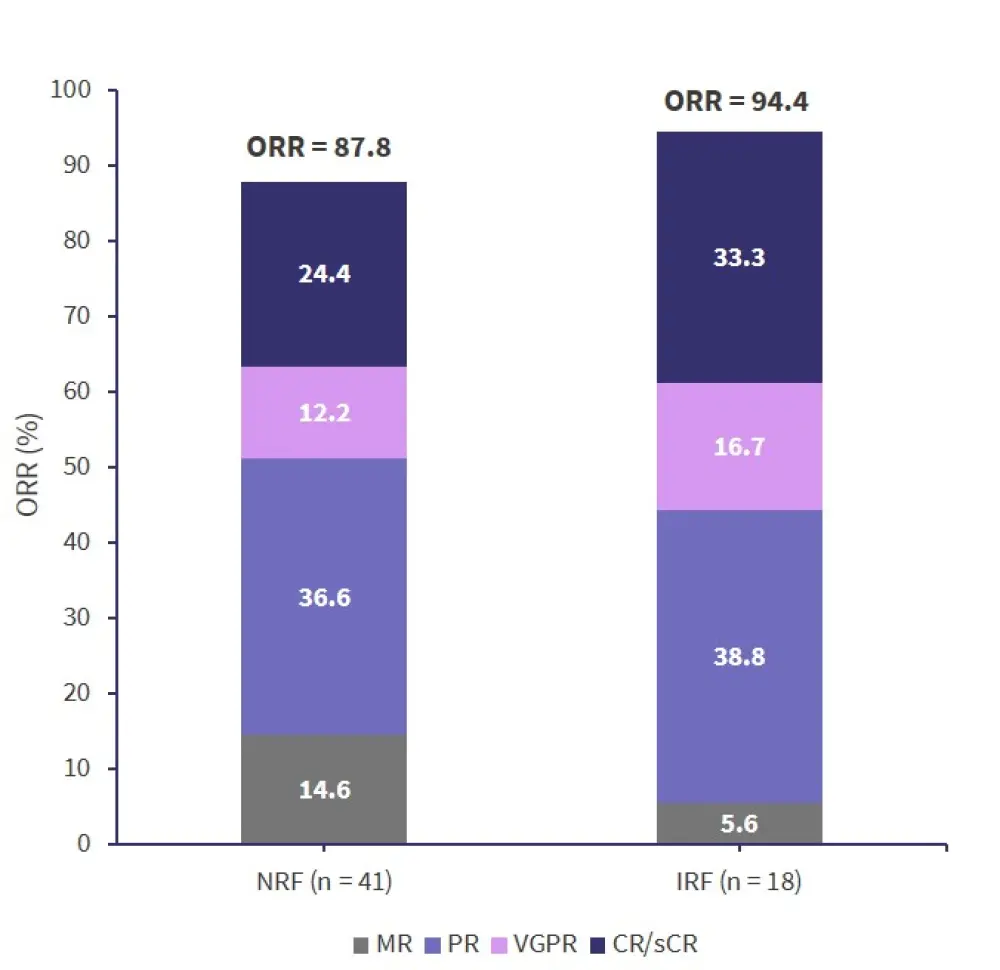
CR, complete response rate; IRF, impaired renal function; MR, minimal response; NRF, normal renal function; ORR, overall response rate; PR, partial response; sCR, stringent complete response; VGPR, very good partial response.
*Data from He, et al.1
The median PFS was:
- 266 days in the NRF group
- 181 in the IRF group (hazard ratio [HR], 0.68; 95% confidence interval [CI], 0.363–1.274; p < 0.05).
Median OS was:
- 877 days in the NRF group
- 238 days in the IRF group (HR, 0.27; 95% CI, 0.140–0.527; p < 0.05).
Effect of CAR T cells on renal function
Blood urea nitrogen and serum creatinine (sCr) were significantly raised in the IRF group compared with the NRF group (p < 0.05). There was also a significant increase in eGFR from baseline in Month 6 after CAR T-cell infusion in the IRF group (p < 0.05). The IRF group saw the greatest decrease in sCr in the first month of treatment, followed by a gradual decline over the next 5 months. Blood urea nitrogen levels remained fairly stable throughout the 12 months since CAR T-cell infusion.
Factors that were found to significantly associated with sCr include:
- light chain type MM (r = 12.33; p = 0.013)
- β-2M (r = 0.268; p < 0.01).
A significant decrease in β-2M was seen in the IRF group following CAR T-cell infusion reaching a maximum in the Month 6 (p < 0.01). sCR was significantly correlated with β-2M (p < 0.01).
Conclusion
The study showed short term improvements in renal function were possible with anti-BCMA CAR T-cell therapy in patients with RRMM. ORR was not significantly different between IRF and NRF patients indicating response to CAR T-cell therapy was not decreased because of poor renal function. However, significant differences were reported in long-term outcomes: OS and progression free survival were decreased in the IRF group compared with the NRF. In addition, a greater proportion of patients in the IRF group had high-risk cytogenetics, which is associated with a poor prognosis than in the NRF group.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?

