All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Recommendations for the management of multiple myeloma-related renal impairment: International Myeloma Working Group
Do you know... Supportive care in patients with multiple myeloma (MM) and renal impairment is important. Which of the following supportive care therapies is not recommended in these updated recommendations for patients with MM and severe renal impairment?
Renal impairment is a common complication of multiple myeloma (MM), affecting up to 50% of patients. Renal impairment is defined as an estimated glomerular filtration rate (eGFR) of <60 mL/min per 1.73 m2. Poor overall survival (OS) and increased risk of early death are associated with renal impairment in patients with MM.
The International Myeloma Working Group (IMWG) recently updated their previous recommendations for the diagnosis and management of renal impairment in patients with MM.1 The recommendations were published by Dimopoulos et al.1 in The Lancet. An interdisciplinary panel comprising clinical experts on MM and renal impairment reviewed and graded the evidence according to Grading of Recommendations, Assessment, Development, and Evaluation system.1 We are pleased to summarize the IMWG recommendations particularly those relating to therapeutic approaches.
IMWG Recommendations
Pathophysiology, diagnosis, and staging recommendations
In patients with MM, residual free light chains (FLCs) in the proximal tubules, other co-existing renal diseases, and non-immunoglobulin-related factors have been found to be associated with renal impairment. Early identification and prompt management of renal impairment at diagnosis and relapse is crucial to improve patient outcomes. Figure 1 shows the algorithm for the differential diagnosis of renal impairment in patients with MM.
Figure 1. Algorithm for differential diagnosis of renal impairment*
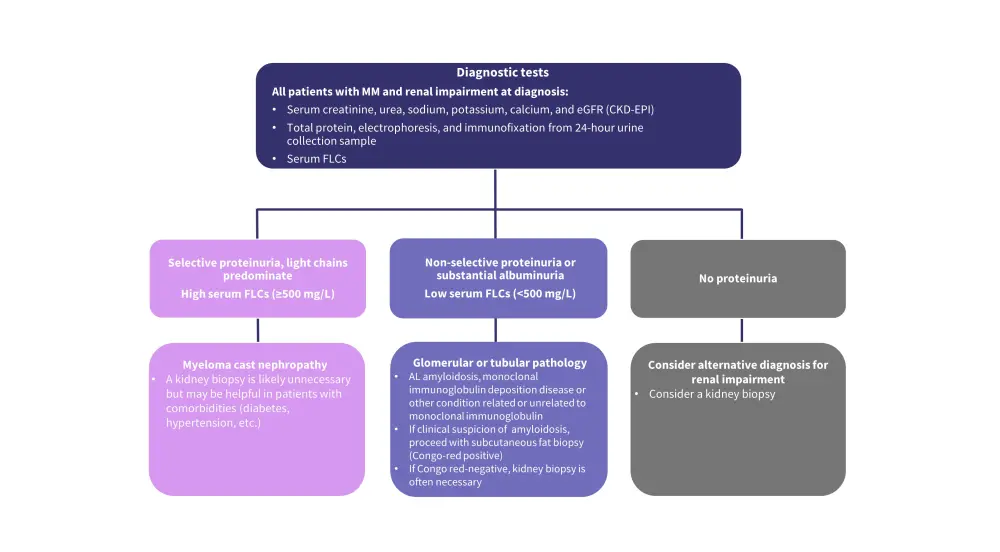
AL, amyloid light-chain; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; eGFR, estimated glomerular filtration rate; FLC, free light chain; MM, multiple myeloma.
*Adapted from Dimopoulos et al.1
- The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula without race variable should be used to calculate eGFR.
- CKD classification should be used for the staging of stable renal impairment (Table 1).
- Patients with acute kidney injury should be staged using the Kidney Disease Improving Global Outcomes (KDIGO), Risk, Injury, Failure, Loss, and End-stage kidney disease (RIFLE), and Acute Kidney Injury Network (AKIN) criteria can also be used.
- β-microglobulin at baseline should be measured in all patients with MM.
Table 1. Staging of chronic kidney disease*
|
eGFR, estimated glomerular filtration rate; RRT, renal replacement therapy. |
||
|
|
Description |
eGFR (mL/min per 1.73 m2) |
|---|---|---|
|
1 |
Normal or elevated eGFR |
≥90 |
|
2 |
Mild reduction in eGFR |
60–89 |
|
3 |
Moderate reduction in eGFR |
30–59 |
|
4 |
Severe reduction in eGFR |
15–29 |
|
5 |
Renal failure or end-stage renal disease |
<15 or RRT |
Criteria for renal response
Reversal of renal impairment should be the main aim of treatment in patients with MM-related renal impairment. Table 2 shows the IMWG criteria for renal response to anti-myeloma treatment and should be used in both clinical trials and practice.
- Specific renal response criteria should be used in the presence of biopsy-confirmed amyloid light-chain amyloidosis.
- Careful evaluation of efficacy and toxicity of chemotherapy should be considered in patients with myeloma cast nephropathy.
Table 2. Criteria for renal response*
|
eGFR, estimated glomerular filtration rate. |
||
|
Responses |
Baseline eGFR (mL/min per 1.73 m2)† |
Best creatinine clearance response (mL/min) |
|---|---|---|
|
Complete response |
<50 |
≥60 |
|
Partial response |
<15 |
30–59 |
|
Minor response |
<15 |
15–29 |
|
15–29 |
30–59 |
|
Supportive care
Adequate and immediate supportive care is important for all patients with MM-related renal impairment. Figure 2 shows the supportive care recommendations.
Figure 2. Supportive care recommendations*
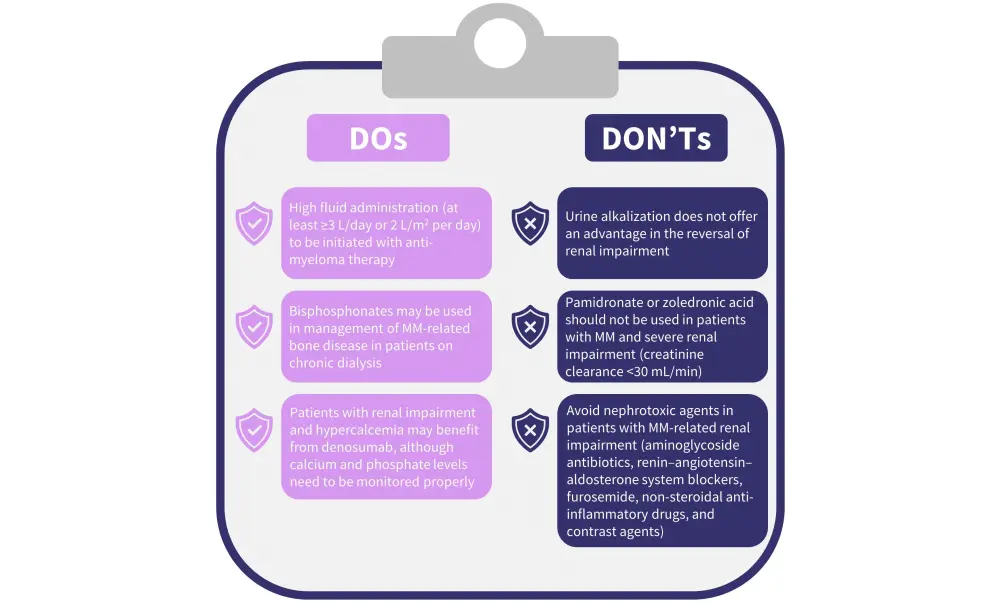
MM, multiple myeloma.
*Adapted from Dimopoulos et al.1
Mechanical approaches
In patients with MM and renal impairment, mechanical approaches have been employed to rapidly decrease the serum FLCs concentrations. In addition, administration of anti-myeloma treatment is also important to reduce the production of monoclonal FLC (Figure 3).
Figure 3. Recommendations for mechanical approaches*
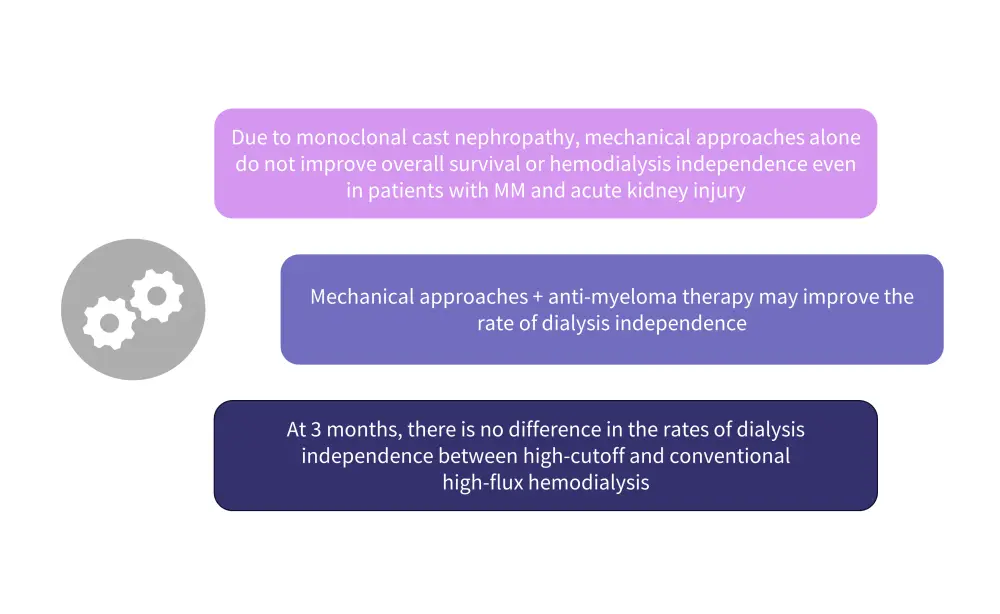
MM, multiple myeloma.
*Adapted from Dimopoulos et al.1
Anti-myeloma therapy
- Patients with MM should receive immediate treatment for renal impairment, as it may be reversible.
- Patients with newly diagnosed MM and renal impairment have a high chance of improvement.
- Patients with relapsed or refractory (R/R) MM and renal impairment tend to be more challenging to manage.
- Bortezomib regimens remain the mainstay of first-line therapy in the management of MM and is also seen as the gold-standard therapy for the treatment of patients with MM and renal impairment.
- Recommendations for high-dose steroids, protease inhibitors, and immunomodulatory drugs are shown in Figure 4
Figure 4. Recommendations for anti-myeloma therapies*

IV, intravenous; MM, multiple myeloma, R/R, relapsed/refractory.
*Adapted from Dimopoulos et al.1
- Monoclonal antibodies, when used in combinations as triplet or quadruplet have enhanced outcomes in patients with relapsed or newly diagnosed MM, respectively (Figure 5).
- Anti-CD38-based triplet combinations in patients with R/R MM and moderate-to-severe renal impairment include:
- Daratumumab–bortezomib– dexamethasone
- Daratumumab–carfilzomib–dexamethasone
- Isatuximab–carfilzomib–dexamethasone
- Anti-CD38-based triplet combinations in patients with R/R MM and moderate renal impairment include:
- Daratumumab–lenalidomide–dexamethasone
- Daratumumab–pomalidomide–dexamethasone
- Isatuximab–pomalidomide–dexamethasone
- Anti-CD38-based triplet combinations in patients with R/R MM and moderate-to-severe renal impairment include:
- In patients with newly diagnosed MM, autologous hematopoietic stem cell transplantation (auto-HSCT) remains a standard of care and may be feasible in patients with stable renal impairment with a potential dose adjustment (Figure 5).
- However, in patients with acute kidney injury, auto-HSCT is not an option.
- Bispecific T-cell engagers offer deep and durable responses in patients with R/R MM.
- Chimeric antigen receptor T-cell targeting B-cell maturation antigen is safe and effective in patients with R/R MM.
- However, there is no dosing recommendation in patients with an eGFR of <30 mL/min per 1.73 m2.
Figure 5. Recommendations for anti-myeloma therapies*

HSCT, hematopoietic stem cell transplantation; MM, multiple myeloma, R/R, relapsed/refractory.
*Adapted from Dimopoulos et al.1
Kidney transplantation
Although kidney transplantation in some patients with MM and end-stage renal impairment who have previously undergone auto-HSCT have shown encouraging results, the best time for transplantation is unknown.
- The recommendation by IMWG is to consider kidney transplantation in referral centers only for patients with end-stage renal impairment who are fit and have sustained MM disease control (minimal residual disease negative status for 2 years).
- A multidisciplinary approach is recommended to manage the adverse events from combined immunosuppressive treatment and anti-myeloma therapy.
Conclusion
These updated recommendations by IMWG address the therapeutic innovations in MM and the introduction of new options for the management of patients with MM and renal impairment. It also reiterates that diagnosis and management of renal impairment in patients with MM is often challenging and requires a multidisciplinary approach. However, the recommendations are limited by variation in renal impairment definition and exclusion criteria in available studies, inappropriate use of formulae for the estimation of renal function in chronic kidney disease in patients with acute kidney injury, potential differential diagnosis of renal impairment in patients with MM, and the lack of prospective data. Future studies should address these limitations to optimize clinical practice and patient outcomes.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?

