All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Prognostic impact of MRD dynamics during maintenance therapy for patients with NDMM
Measurable residual disease (MRD) assessment is a key prognostic tool for patients with newly diagnosed multiple myeloma.1 There is now growing potential for the use of serial MRD analysis rather than a one-off assessment to guide treatment decisions, particularly in relation to maintenance therapy and observation scenarios.1 Relevant data surrounding these clinical settings however remains limited.1
In order to bridge this knowledge gap, Bruno Paiva et al.1 conducted a pooled analysis of two phase III trials investigating ixazomib maintenance therapy for patients with newly diagnosed multiple myeloma. The aim of the analysis was to evaluate the progression-free survival (PFS) in relation to MRD status, the variability of MRD status over time and its impact, and the PFS benefit with ixazomib maintenance compared with placebo.1 We summarize the findings in the article below.
Study design
The two randomized phase III trials chosen for the pooled analysis were the TOURMALINE-MM3 (NCT02181413) and -MM4 (NCT02312258) studies. For more information on previous results from these trials, check out this Multiple Myeloma Hub article.
Both had similar study designs and were conducted in over 30 countries and across more than 160 clinical sites.
The study protocol for the MM3 and MM4 trials was as follows:
- Patients randomized at a 3:2 ratio (maintenance vs placebo)
- Ixazomib: Oral, 3 mg on Days 1, 8, and 15 (28-day cycle)
- Ixazomib dose increased to 4 mg from Cycle 5 if tolerated in previous cycles
- Placebo therapy matched ixazomib
- Bone marrow aspirates taken at randomization, Cycle 13, and at the end of treatment were used to assess patient MRD by eight-color flow cytometry
The primary endpoint for both studies was PFS. Secondary endpoints included the frequency of conversion from positive to negative MRD, sustained MRD negativity, and the correlation between MRD status and survival.
Results
A total of 1,362 patients were included in the pooled analysis and MRD status was available in 1,280 patients at the beginning of randomization:
- 20.5% had undetectable MRD
- 79.5% had detectable MRD
Patient characteristics, not including age, were generally well-balanced between patients with negative MRD and those with positive MRD at randomization before maintenance therapy (Table 1).
Table 1. Patient characteristics*
|
CR, complete response; IMiD, immunomodulatory drug; ISS, International Scoring System; MRD, measurable |
||||
|
Characteristic, % (unless otherwise stated) |
MRD negative |
MRD positive |
||
|---|---|---|---|---|
|
Ixazomib |
Placebo |
Ixazomib |
Placebo |
|
|
Patients, n |
161 |
101 |
606 |
412 |
|
Age |
||||
|
<75 years |
90.1 |
90.1 |
77.6 |
76.2 |
|
≥75 years |
9.9 |
9.9 |
22.4 |
23.8 |
|
Sex |
||||
|
Male |
58.4 |
59.4 |
58.1 |
59.0 |
|
Female |
41.6 |
40.6 |
41.9 |
41.0 |
|
Cytogenetic abnormalities at diagnosis |
||||
|
High risk† |
13.0 |
26.7 |
17.2 |
17.0 |
|
Corresponding standard-risk |
64.0 |
54.5 |
65.2 |
64.3 |
|
Unclassifiable |
23.0 |
18.8 |
17.7 |
18.7 |
|
Expanded high risk‡ |
26.1 |
36.6 |
33.7 |
32.8 |
|
Corresponding standard risk |
40.4 |
34.7 |
36.8 |
36.2 |
|
Unclassifiable |
33.5 |
28.7 |
29.5 |
31.1 |
|
Preinduction ISS stage |
||||
|
I or II |
67.1 |
68.3 |
68.5 |
67.2 |
|
III |
32.9 |
31.7 |
31.5 |
32.8 |
|
PI exposed |
90.1 |
89.1 |
84.3 |
83.0 |
|
IMiD exposed |
39.8 |
43.6 |
34.3 |
35.0 |
|
Response status after transplant |
|
|
|
|
|
CR or VGPR |
95.0 |
92.1 |
61.1 |
63.6 |
|
PR |
5.0 |
7.9 |
38.9 |
36.4 |
MRD status at randomization
Regardless of MRD status, patients treated with ixazomib experienced a higher median PFS than placebo (Figure 1). Overall, the median PFS for patients who were MRD negative was 38.6 months compared with 15.6 months for patients who were MRD positive (hazard ratio [HR], 0.47; 95% confidence interval [CI], 0.37–0.58; p = 0.019). Negative MRD status was associated with prolonged PFS in nearly all patient subgroups, including cytogenetic risk and prior treatment. Moreover, patients had a greater reduction in the risk of progressive disease (PD) or death if they were either enrolled in the MM4 trial, ≥75 years old, or had an International Staging System score of 3.
Figure 1. Median PFS for patients treated with ixazomib maintenance or placebo by MRD status at randomization*
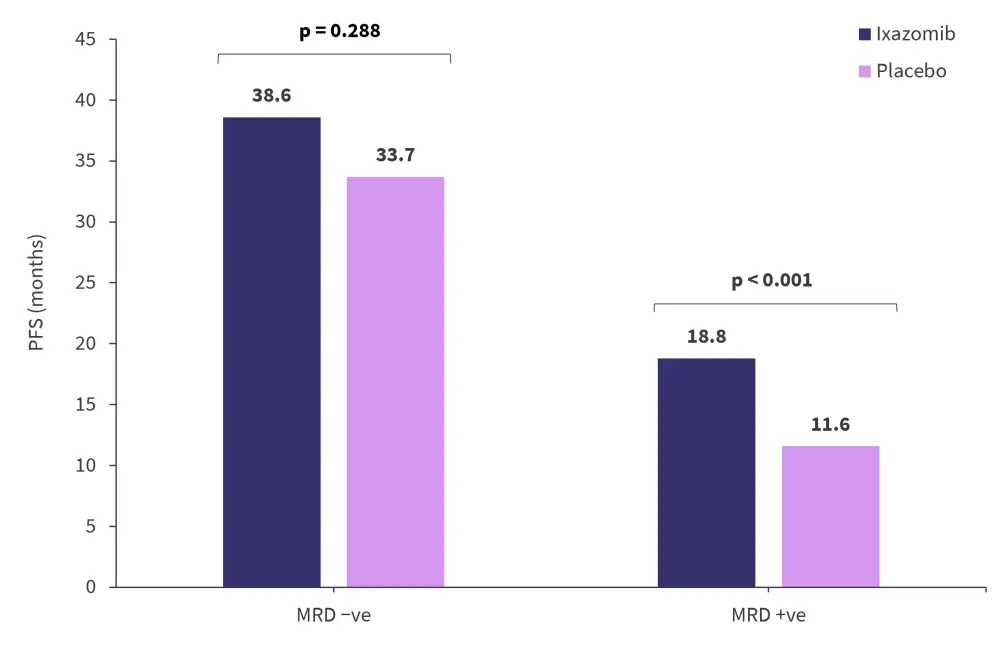
MRD, measurable residual disease; PFS, progression-free survival.
*Adapted from Paiva, et al.1
MRD dynamics
The evaluation of MRD dynamics was achieved by paired assessments at randomization and during maintenance. A total of 1,166 patients were analyzed and found that almost half of MRD-negative patients at randomization lost their negative status in <2 years (111/229, 48.5%). In contrast, a small group of patients who were MRD positive converted to MRD negative status during maintenance therapy (60/937, 6.4%). The MRD dynamics of patients during the maintenance period regardless of therapy is shown in Figure 2.
Figure 2. MRD dynamics during maintenance regardless of treatment*
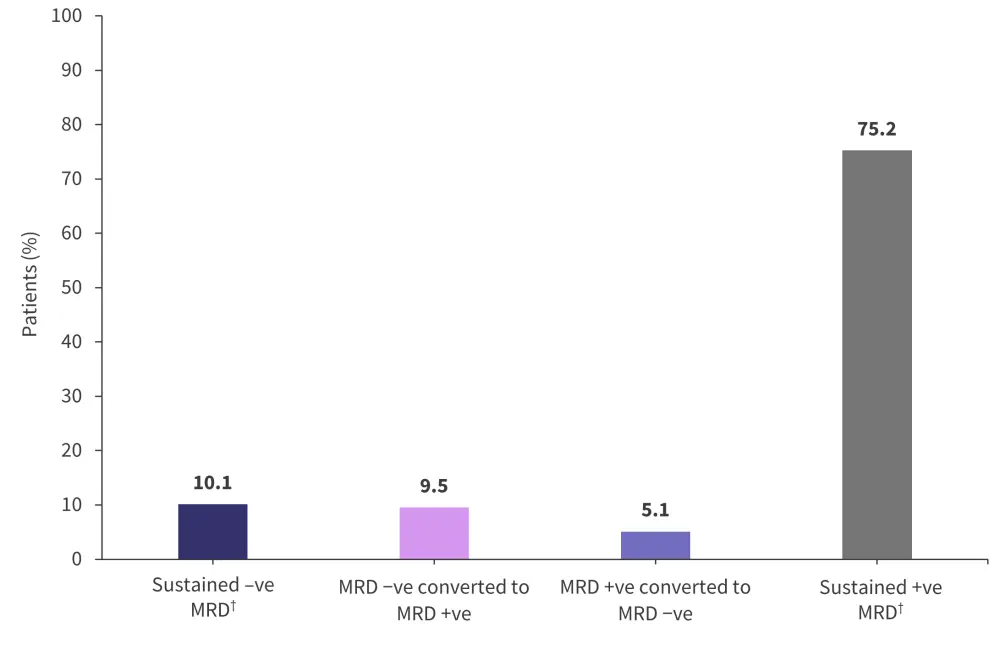
MRD, measurable residual disease.
*Adapted from Paiva, et al.1
†Sustained MRD was defined as a positive or negative status held for more than 12 months.
The 14-month landmark analysis showed 2-year PFS rates to be higher in patients who had either converted to negative MRD or had sustained negative MRD status (Figure 3).
Figure 3. 14-month analysis of 2-year PFS rates according to MRD dynamics regardless of treatment*
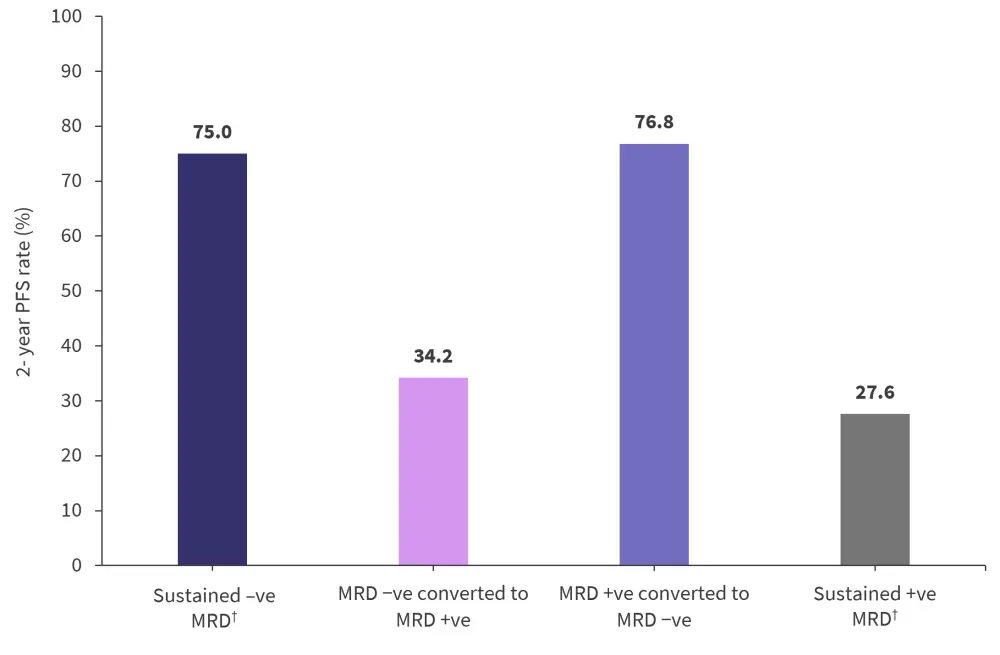
MRD, measurable residual disease; PFS, progression-free survival.
*Adapted from Paiva, et al.1
†Sustained MRD was defined as a positive or negative status held for more than 12 months.
The risk of PD or death increased for patients who had converted from negative to positive MRD status compared with patients with sustained negative MRD (HR, 3.31; 95% CI, 1.77–6.20; p < 0.001). This was the same for patients with persistent positive MRD compared with those who had converted from positive to negative status (HR, 3.72; 95% CI, 1.85–7.46; p < 0.001).
Impact of ixazomib maintenance and placebo on MRD positive and negative patients
At the 14-month landmark analysis, patients treated with ixazomib showed a statistically significant difference in the rates of sustained MRD negativity compared with placebo at 76% and 60.3%, respectively (p = 0.04). There were no differences in the rate of conversions from MRD positive to MRD negative status.
At the 14-month landmark analysis, the 2-year PFS for patients who were MRD negative was higher compared with patients who were MRD positive (Figure 4). Higher rates of PFS were also observed with ixazomib in patients whose MRD levels had not increased from randomization to the 14-month analysis timepoint (HR, 0.39; 95% CI, 0.22–0.68; p < 0.001) compared with patients whose MRD level had increased (HR, 0.67; 95% CI, 0.22–2.07; p = 0.487). In conjunction with the marginal differences in the rate of conversions from MRD positive to MRD negative status with ixazomib versus placebo, these findings suggest that rather than erasing MRD, treatment was able to control the size of the clone for longer periods.
Figure 4. 14-month analysis of 2-year PFS rates in patients who were MRD positive or negative and treated with either ixazomib or placebo*
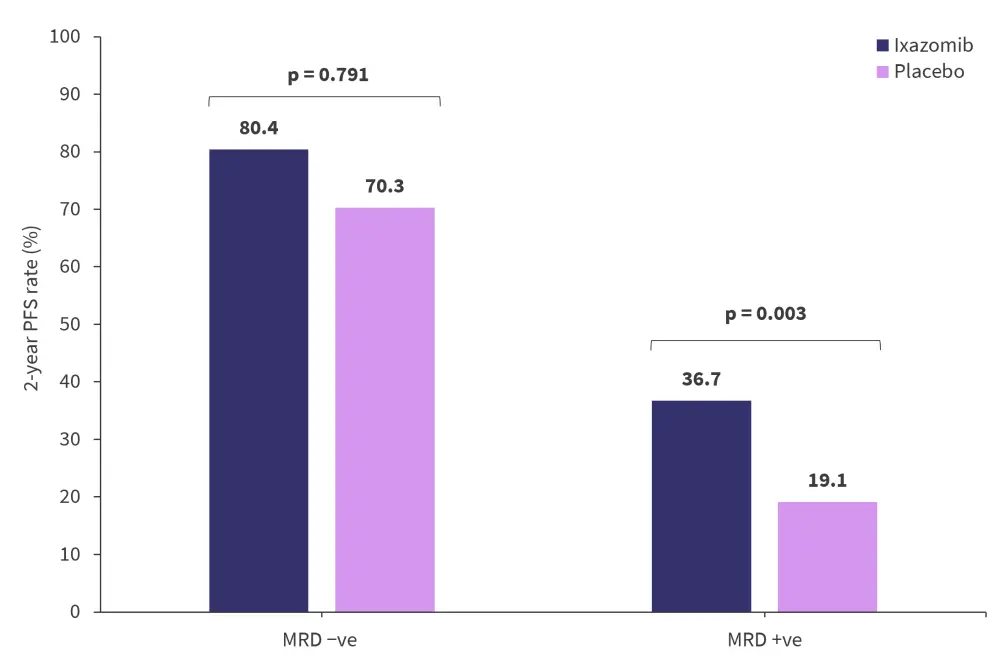
MRD, measurable residual disease; PFS, progression free survival.
*Adapted from Paiva, et al.1
Future directions2
While this analysis provides many insights into the use of MRD to guide treatment decisions, especially in the maintenance setting, there remain several key areas for further investigation:
- Can we shorten maintenance duration in patients with sustained MRD negativity?
- Should we escalate therapy for patients with an MRD-positive status to convert to a negative MRD?
- Can blood-based MRD assays facilitate earlier detection of change in MRD?
- How do these marrow and blood dynamics correlate with imaging?
- Can we predict which patients will convert from MRD negative to positive?
- Should serial MRD assessment be prioritized over a one-off evaluation?
Conclusion
Results from this analysis highlight that MRD negative status is associated with prolonged PFS compared with patients who are MRD positive in almost all patient subgroups. Treatment with ixazomib was found to improve the PFS in patients with an MRD-positive status both at randomization and the 14-month landmark time point. However, 2-year ixazomib maintenance treatment was not effective in patients who were MRD negative at randomization or at the 14-month landmark. Interestingly, MRD status before maintenance therapy was found to be an independent prognostic factor for PFS. The clinical implications of MRD status over time are yet to be fully established; however, PFS results from this study indicate that MRD negativity as an endpoint for treatment could be extrapolated to a maintenance setting. Furthermore, persistent MRD was consistently associated with PD, and therefore, it is reasonable to suggest acting upon an MRD-positive status with treatment intensification rather than a negative MRD status.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?

