All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Phase I results of MEDI2228, an anti-BCMA antibody–drug conjugate, in patients with RRMM
The expression of B-cell maturation antigen (BCMA) occurs at all stages of multiple myeloma (MM); promisingly, BCMA is a tumor-specific antigen that can be targeted repeatedly. MEDI2228 is an antibody–drug conjugate (ADC) that targets the extracellular domain of human BCMA and preferentially binds to membrane-bound BCMA versus soluble BCMA. The ADC is linked to a DNA cross-linking pyrrolobenzodiazepine dimer. It is currently under investigation in relapsed/refractory MM (RRMM), and phase I results were presented by Multiple Myeloma Hub Steering Committee member, Shaji K. Kumar, during the 62nd American Society of Hematology (ASH) Annual Meeting and Exposition.
The first-in-class humanized anti-BCMA ADC, belantamab mafodotin, was approved in 2020 by both the U.S. Food and Drug Administration (FDA) and the European Commission for the treatment of adult patients with RRMM who received ≥ 4 therapies and progressed on the last treatment.
Study design
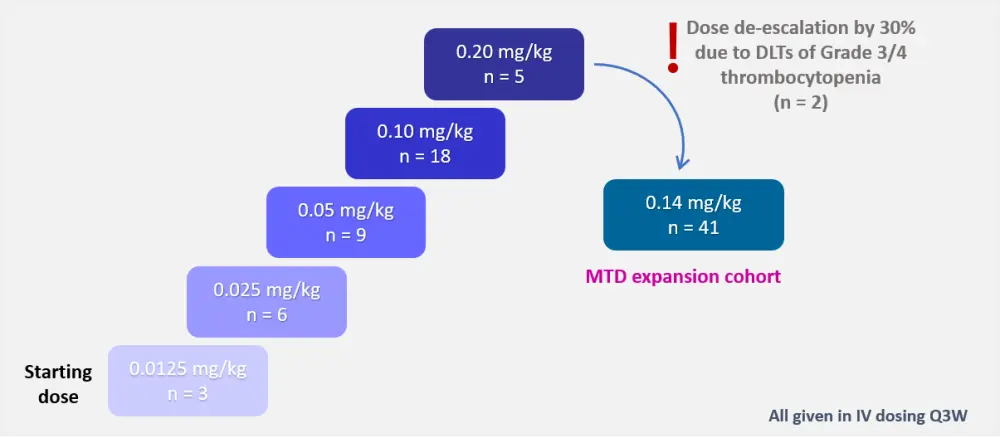
- A first-in-human, open-label, phase I study (NCT03489525)
- Dose-escalation design (Figure 1)
- Eligibility criteria:
- Aged ≥ 18 years
- Confirmed and measurable RRMM
- European Cooperative Oncology Group (ECOG) performance status ≤ 1
- Disease progression following treatment with proteasome inhibitors (PIs), immunomodulatory drug (IMIDs), and monoclonal antibodies (mAbs)
- Primary endpoints included safety and tolerability
- Secondary endpoints were preliminary efficacy, pharmacokinetics, and immunogenicity
Figure 1. Dose-escalation design1
Patients
Total number of patients included in the study was 82, with a median age of 69 years (range, 40–89). More than half of patients were triple-refractory by the time of enrolment. Baseline characteristics are provided in Table 1.
Table 1. Baseline characteristics in MTD expansion cohort (0.14 mg/kg) and study population1
|
Characteristic |
0.14 mg/kg cohort |
Total |
|---|---|---|
|
IMID, immunomodulatory imide drug; mAb, monoclonal antibody; MTD, maximum tolerated dose; PI, proteasome inhibitor. |
||
|
Age, years (range) |
69 (40–85) |
69 (40–89) |
|
Prior therapies, % |
||
|
1–4 regimens |
48.8 |
36.6 |
|
5–7 regimens |
29.2 |
41.5 |
|
≥ 8 regimens |
22.0 |
22.0 |
|
IMIDs |
100 |
98.8 |
|
PIs |
100 |
100 |
|
mAbs |
97.6 |
97.6 |
|
Triple-refractory, % |
56.1 |
57.3 |
Results
At the data cutoff date, all patients in the maximum tolerated dose (MTD) expansion cohort discontinued treatment. This was mainly due to adverse events (AEs; n = 27), followed by disease progression (n = 10), patient decision (n = 2), investigator decision (n = 1), and death (n = 1). Of note, the most common treatment-related AE (TRAE) leading to discontinuation was photophobia (27%).
Safety
TRAEs were in line with the expected toxicity of the pyrrolobenzodiazepine drug class and mostly Grade 1/2 in severity. Grade 3/4 TRAEs of interest included photophobia (not a class-related AE), thrombocytopenia, pleural effusion, and increased gamma-glutamyltransferase (Table 2).
Table 2. Grade 3/4 TRAEs among different dose levels1
|
AE, % |
0.025 mg/kg |
0.05 mg/kg |
0.10 mg/kg |
0.14 mg/kg |
0.20 mg/kg |
|---|---|---|---|---|---|
|
AE, adverse event; GGT, gamma-glutamyltransferase; TRAE, treatment-related AE. |
|||||
|
Photophobia |
0 |
22.2 |
11.1 |
17.1 |
0 |
|
Thrombocytopenia |
0 |
0 |
11.1 |
24.4 |
60.0 |
|
Pleural effusion |
0 |
0 |
11.1 |
2.4 |
0 |
|
GGT increased |
16.7 |
11.1 |
0 |
19.5 |
0 |
Ocular toxicity
Oculotoxic events, specifically photophobia, were considered an unexpected event, not treatment-related, based on previous experience. Photophobia of any grade was reported in a total of 36 patients, including the 58.5% of patients enrolled in the 0.14 mg/kg dose level cohort.
Patients experienced increased tearing, blurry vision, and swelling. The median time to onset of these events was about 2 months or 2–3 cycles of treatment. Photophobia improved upon discontinuation in most patients and resolved in four patients; however, some patients were lost to follow-up. The pathophysiology of photophobia remains unknown.
No keratopathy was reported in patients who experienced photophobia in the MTD expansion cohort (0.14 mg/kg).
Efficacy
Responses were observed at all dose levels of MEDI2228. Overall response rate (complete response plus partial response) was highest in the MTD expansion cohort (65.9%; 95% CI, 49.4–79.9) versus other dose levels (≤ 40%). Amongst 23 patients who were triple-refractory to PIs, IMIDs, and mAbs, 17 achieved a partial response or better. The best overall response rates in the MTD expansion cohort are detailed in Figure 2.
Figure 2. Best overall response (N = 41)1
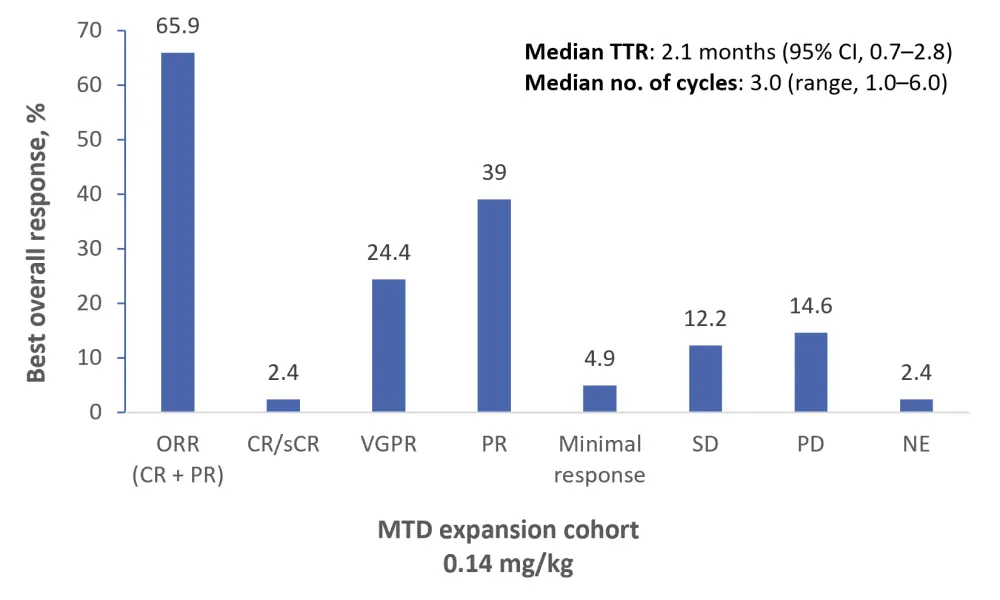
Median duration of response was 5.9 months in the MTD expansion cohort; however, it was acknowledged that this could be underestimated due to loss to follow-up. Deep, durable responses were observed even weeks after treatment discontinuation.
Pharmacokinetics
There was a proportional increase in exposure at dose levels ≥ 0.05 mg/kg. Dosing at 3-week intervals (Q3W) did not lead to significant accumulation. The impact of baseline-soluble BCMA levels on pharmacokinetics was minimal, highlighting the specificity of binding to membrane-bound BCMA.
Conclusion
These results suggest that MEDI2228 had a manageable safety profile, with clinical efficacy among several dose levels in a heavily pretreated patient population with RRMM. Thrombocytopenia led to dose limitations in two patients, and 0.14 mg/kg Q3W was selected as the MTD. Photophobia was an unexpected event and led to treatment discontinuations; however, improvements were seen with the discontinuation of MEDI2228. Expansion cohorts with alternative doses and schedule to 0.14 mg/kg Q3W are ongoing with a special interest in ocular toxicity.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?

