All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Opportunistic infections in multiple myeloma part II: Prophylaxis and management
Do you know... What vaccination is recommended at Day 50–70 after autologous or allogeneic HSCT?
Infections are one of the most prolific causes of mortality and morbidity in patients with multiple myeloma (MM), second only to the disease itself. Infections are also significantly more common in patients with MM, with certain treatments and disease stages posing a particularly high-risk; for instance, in the first three months after diagnosis, and during treatment of relapsed/refractory MM. Therefore, there is a need for effective prophylaxis, management, and vaccination in these patients.1
In part I of this article series, we covered the incidence and etiology of opportunistic infections in multiple myeloma. Here, we summarize the latest recommendations for prophylaxis and management of infections.
Vaccination2
Vaccination is one of the most utilized and successful tools to reduce the incidence of serious infection, globally. It is particularly important for patients with MM, who are statistically more likely to acquire infections that result in hospitalization and death. Patients with MM may require a broader spectrum of vaccines and more doses to maintain immunity. Figure 1 outlines the recommendation for vaccination in patients with MM.
Figure 1. Recommendations for vaccination in patients with MM*
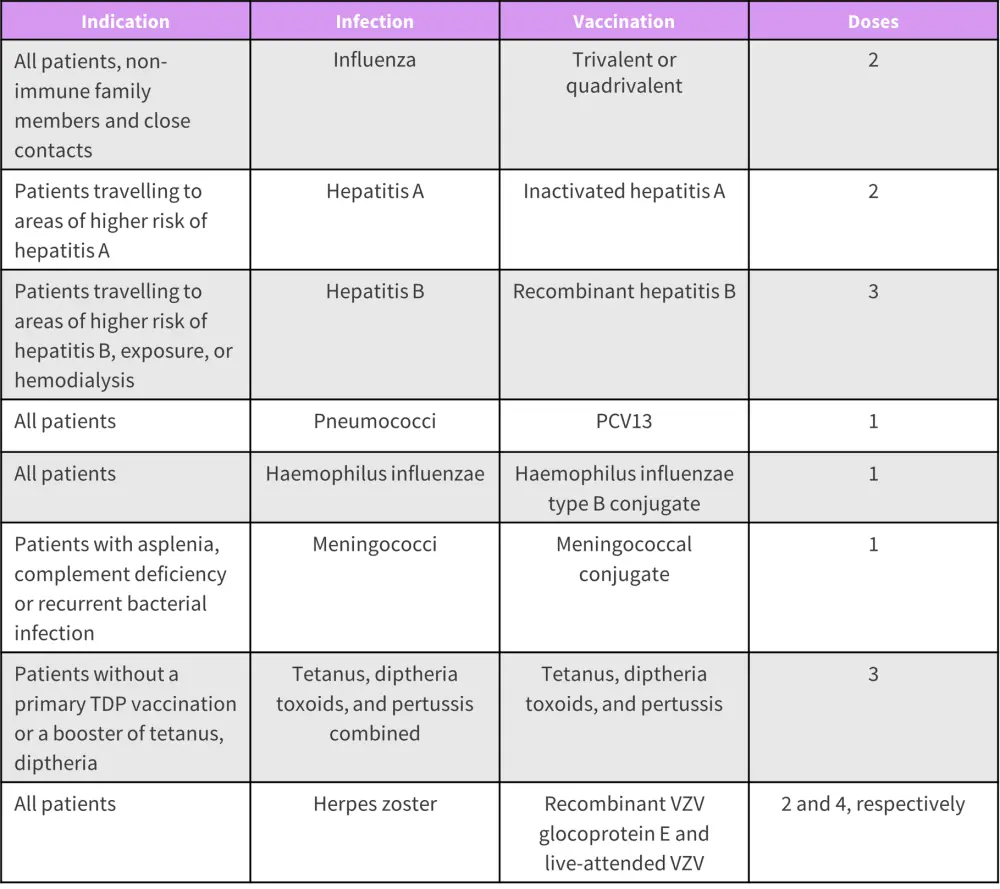
PCV, pneumococcal conjugate vaccine; TDP, tetanus diphtheria pertussis; VZV, varicella zoster
*Adapted from Ludwig, et al.2
After autologous and especially allogenic hematopoietic stem cell transplantation, patients are at a particularly high risk for infection therefore a broad range of vaccinations are recommended following transplant. The recommended vaccination schedule post-hematopoietic stem cell transplantation is shown in Figure 2.
Figure 2. Recommended vaccination schedule after autologous or allogeneic HSCT*
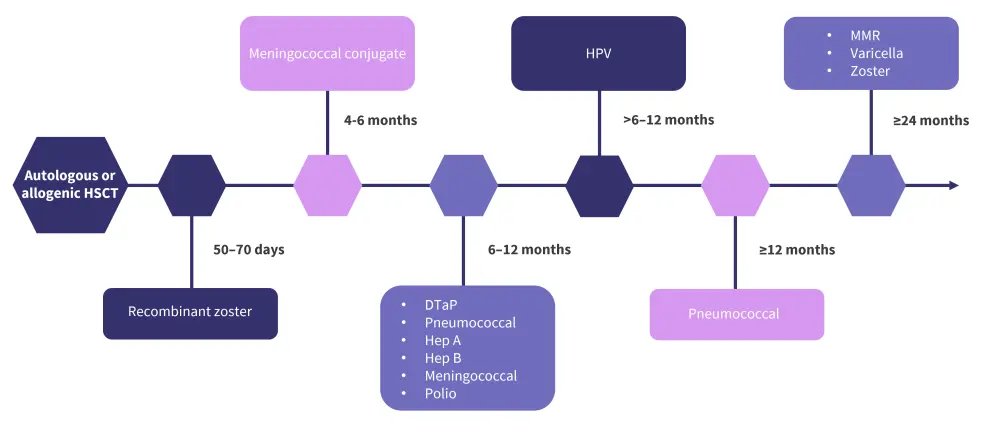
DTaP, diphtheria, tetanus, and pertussis; Hep, hepatitis; HSCT, hematopoietic stem cell transplantation; HPV, human papillomavirus; MMR, measles mumps rubella.
*Data from Ludwig, et al.2
Prophylaxis3
Certain patient populations and treatments place patients at a higher risk of infection. These groups include newly diagnosed patients initiating therapy, those receiving immunosuppressive agents, and those who are severely immunosuppressed following transplant. Other special situations may include those at an increased risk of contracting COVID-19, and patients experiencing recurrent infections with a suboptimal response to antibiotics.
Recommendations for prophylaxis in special situations are summarized in Figure 3.
Figure 3. Prophylactic agents by indication*
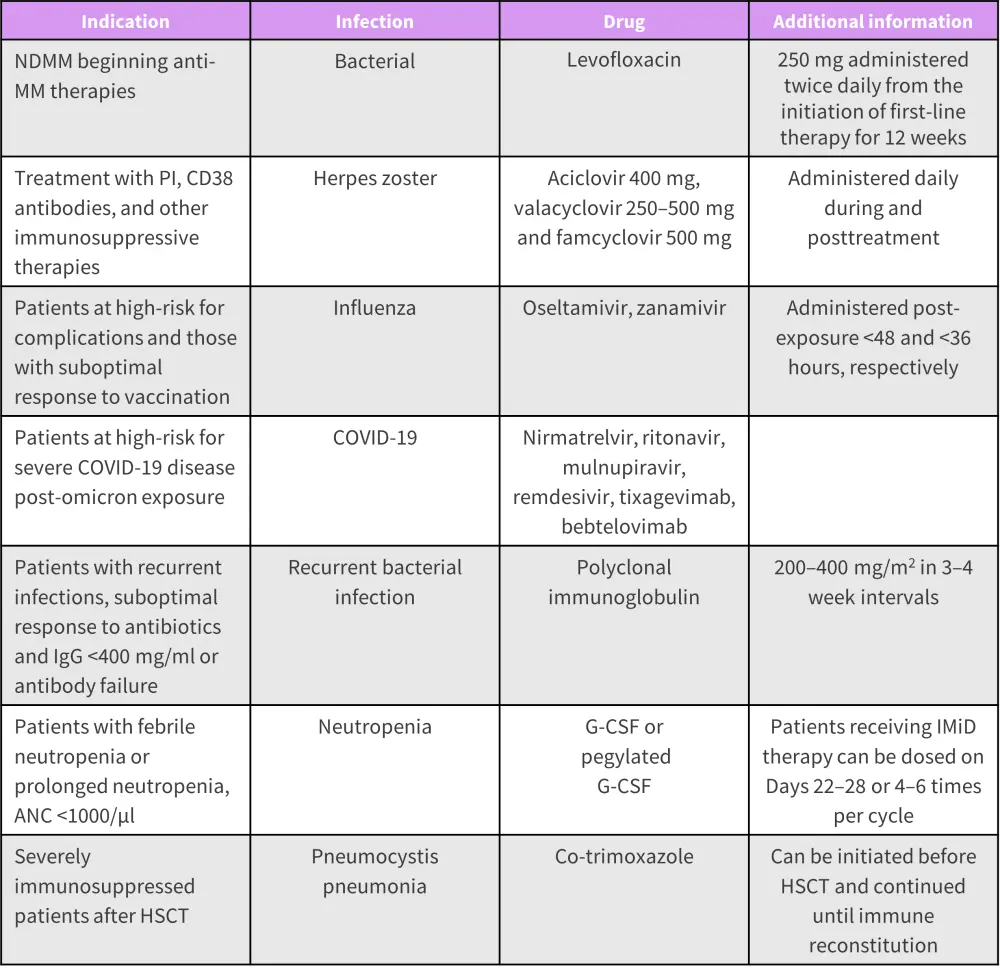
ANC, absolute neutrophil count; G-CSF, granulocyte colony stimulating factor; HSCT, hematopoietic stem cell transplantation; IgG, immunoglobulin G; IMiD, immunomodulatory agent; MM, multiple myeloma; NDMM, newly diagnosed multiple myeloma; PI, proteosome inhibitors.
*Data from Ludwig and Kumar.3
Intravenous immunoglobulin therapy (IVIG)4
IVIG therapy involves administration of antibodies collected from donors, with the aim of replenishing IgG antibody levels in the recipient. IVIG therapy is indicated in patients with MM experiencing a suboptimal response to antibiotics with IgG levels <400 mg/ml or with antibody failure.
Among patients with MM, nearly 40% of infections result in hospitalization. In a single center retrospective study of 108 patients, IVIG treatment was found to be associated with a reduction in infection‐related hospitalizations, as shown in Figure 4.
Figure 4. Risk of hospitalization following infection*
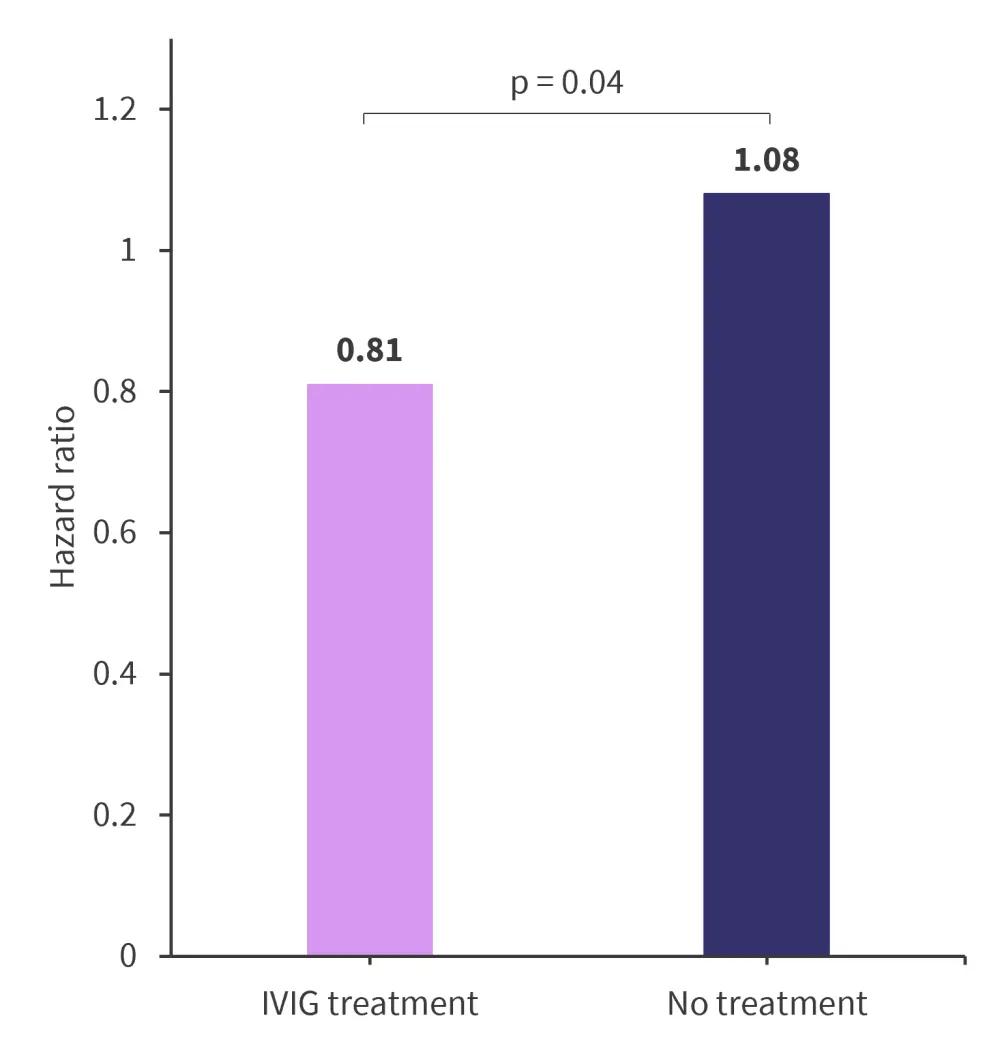
IVIG, intravenous immunoglobulin therapy.
*Data from Sheu, et al.4
IVIG is particularly beneficial in patients treated with B-cell maturation antigen targeted bispecific antibodies. In a retrospective study of 34 patients, the incidence of Grade ≥3 infections was observed to be 90% lower than those not receiving IVIG (p = 0.0307), indicating its use to reduce serious infection and subsequently mortality rates.5 During the 20th International Myeloma Society Annual Meeting 2023, Krina Patel shared her insight on how IVIG therapy be used to treat infection after BCMA-targeted therapies.
How might IVIG therapy be used to treat infection after BCMA-targeted therapies in MM?
Conclusion
Prior to initiating active treatment for MM, an infectious risk assessment should be performed to identify patients who could benefit from early intervention. When selecting prophylactic measures, performance status, past medical history, clinical risk factors and screening for infections should be considered. Vaccination history and risks for common infections such as COVID-19 should be examined on an individual basis. In addition to patients with MM, there may be consideration for vaccination and measures to reduce exposure to family and caregivers.
Clear guidelines are lacking for the identification of patients with MM and high-risk for infections, for appropriate interventions, and further research is required to better inform prophylactic strategies.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?

