All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Novel treatment strategies for light-chain amyloidosis: Updates from EHA 2023
Do you know... CAEL-101 is a novel, chimeric monoclonal antibody that targets fibrils directly. In the open-label, phase II trial described here, what percentage of cardiac evaluable patients experienced a response to CAEL-101 treatment?
Light-chain amyloidosis (AL amyloidosis) is the most common type of systemic amyloidosis caused by plasma cell dyscrasia and is characterized by a build-up of immunoglobulin light chains, which will progressively generate amyloid fibril deposits in various organ sites, including the heart.1,2 These deposits cause organ failure and, eventually, death.2 The current standard-of-care regimen is aimed at targeting the malignant plasma cell to reduce the formation of these fibrils.1 However, there are no current therapies that remove existing deposits from organs.2
CAEL-101 is a novel, chimeric monoclonal antibody that targets fibrils directly and has the potential to remove them from organ sites. Belantamab mafodotin targets B-cell maturation antigen plasma cells expressed in AL amyloidosis. During the European Hematology Association Congress 2023, Michaela Liedtke presented the results from a phase II trial of CAEL-101 in patients with AL amyloidosis,1,2 and Efstathios Kastritis shared the findings from a phase II trial of belantamab mafodotin.3 We are pleased to summarize the key data from these trials here.
CAEL-101 – phase II trial1
- This is an open-label, multicenter, phase II trial (NCT04304144) evaluating the safety and tolerability of CAEL-101 in 25 patients with AL amyloidosis.
- Patients were administered ≤1,000 mg/m2 of CAEL-101 together with cyclophosphamide + bortezomib + dexamethasone -/+ daratumumab in part A of the study.
- Part B of the study consisted of CAEL-101 and anti-plasma cell dyscrasia therapy together with daratumumab treatment.
- Cardiac response was defined as a 30% decrease in N-terminal pro-brain natriuretic peptide or >300 ng/L decrease if baseline levels were ≥650 ng/L.
- Baseline patient characteristics are shown in Table 1.
Table 1. Baseline patient characteristics*
|
Characteristic (% unless otherwise stated) |
N = 25 |
|---|---|
|
Median age, years |
65.2 |
|
Male |
72 |
|
Race |
|
|
White/Caucasian |
92 |
|
Black/African American |
8 |
|
Mayo stage at screening |
|
|
I |
8 |
|
II |
76 |
|
IIIa |
16 |
|
Patients with cardiac involvement |
88 |
|
Prior anti-PCD therapy |
80 |
|
PCD, plasma cell dyscrasia. *Adapted from Valent.1 |
|
Efficacy
- After 18 months of treatment, 23% of cardiac evaluable patients experienced a response (Figure 1).
- One patient experienced cardiac disease progression.
- Responses in patients were observed even after anti-plasma cell dyscrasia therapy cessation.
Figure 1. Response rates in patients receiving CAEL-101 therapy after 18 months*
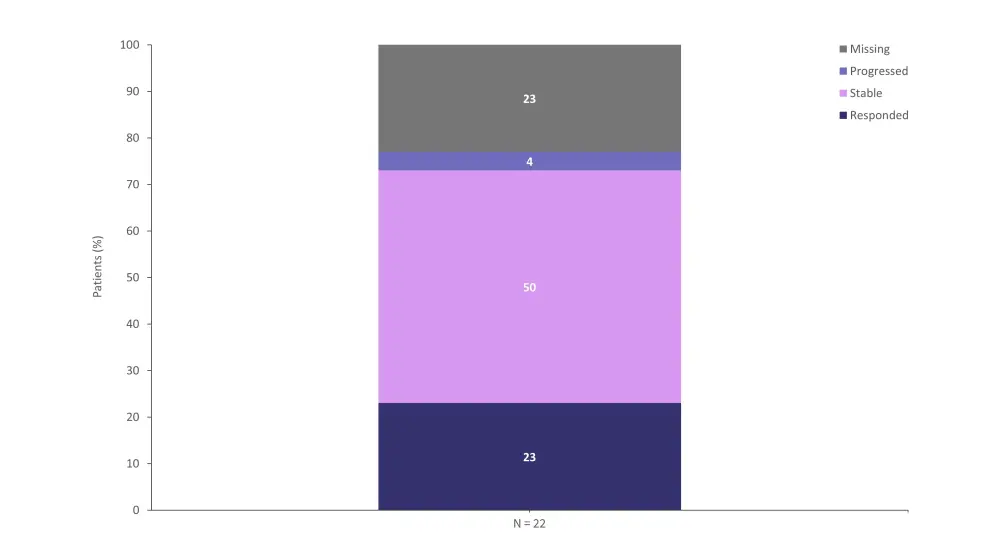
*Adapted from Liedtke.1
Safety
- All patients experienced ≥1 treatment-emergent adverse event (TEAEs).
- 24% of TEAEs were possibly related to treatment.
- ≥60% of TEAEs were Grade ≥3.
- ≥52% of TEAEs were serious adverse events (SAEs).
- Nine patients discontinued the study; all discontinuations were considered unrelated to treatment.
- Adverse events (AEs) of any grade experienced by ≥25% of patients are shown in Figure 2.
Figure 2. Adverse events of any grade experienced by ≥25% of patients treated with CAEL-101*
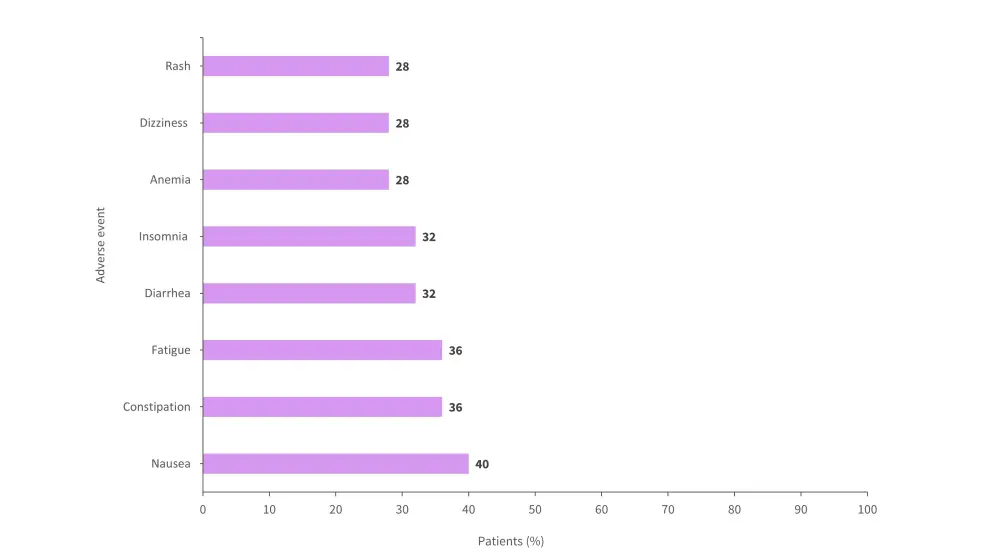
*Adapted from Liedtke.1
Ongoing phase III trials with CAEL-1012
- These are two ongoing international, multicenter, double-blind, randomized, phase III trials (NCT04512235) and (NCT04504825), with the aim to investigate CAEL-101 in combination with standard-of-care chemotherapy in treatment-naïve patients with cardiac AL amyloidosis in Mayo Stages IIIb or IIIa.
- 267 and 124 patients with amyloidosis Stage IIIa are currently enrolled in trial NCT04512235 and NCT04504825, respectively.
- Eligible patients are randomized 2:1 to receive 1,000 mg/m2 of CAEL-101 intravenously once weekly or placebo for 4 weeks, followed by maintenance dosing every 2 weeks.
- The primary endpoint in both trials is overall survival.
- Secondary endpoints include functional outcomes, quality of life and echocardiography.
Conclusion
The phase II trial showed that CAEL-101 was well tolerated among patients with AL amyloidosis and presented no evidence of organ toxicity. Most TEAEs were mild or moderate in severity. Further data on the efficacy and safety of CAEL-101 will become available from the two ongoing phase III trials.
Belantamab mafodotin3
- This is an open-label, multinational, phase II trial assessing the efficacy and safety of belantamab mafodotin in patients with relapsed or refractory AL amyloidosis.
- Eligible patients had Mayo Stage I–IIIA.
- Patients were administered 2.5 mg/kg of intravenous belantamab mafodotin monotherapy every 42 days for up to 8 cycles.
- The primary objective of the study was to present an interim efficacy and safety analysis of belantamab mafodotin from the prospective EMN27 study.
- Secondary endpoints included the safety profile of patients, secondary efficacy evaluations, and characterization of the pharmacokinetic profile.
- Full baseline patient characteristics are shown in Table 2.
Table 2. Baseline patient characteristics*
|
dFLC, difference between involved free light-chain and uninvolved free light-chain; NT-proBNP, N-terminal pro-brain |
|
|
Characteristic (% unless otherwise stated) |
N = 25 |
|---|---|
|
Median age, years (range) |
66 (46–80) |
|
Sex |
|
|
Male |
60 |
|
Female |
40 |
|
NYHA class |
|
|
I |
24 |
|
II |
76 |
|
NT-proBNP, ng/L |
1,197 |
|
Median dFLC, mg/L (range) |
117.1 (37.8–2791) |
|
Median number of previous treatments, n (range) |
3 (1–10) |
|
Prior treatments |
|
|
Daratumumab |
72 |
|
Borterzomib |
92 |
|
Organ involvement |
|
|
Heart |
76 |
|
Kidney |
64 |
|
Peripheral nervous system |
24 |
|
Liver |
16 |
|
Soft tissue |
20 |
|
GI tract |
12 |
Efficacy
- At a median follow up time of 11 months, 25 patients were included, of whom 18 were previously treated with daratumumab.
- The overall hematological response rate for all patients was 60%, and 36% of all patients experienced a very good partial response (Figure 3).
- The overall hematologic response rate for patients with prior daratumumab exposure was 56%, and 38.9% experienced a very good partial response (Figure 3).
Figure 3. Response rates of patients treated with belantamab mafodotin*
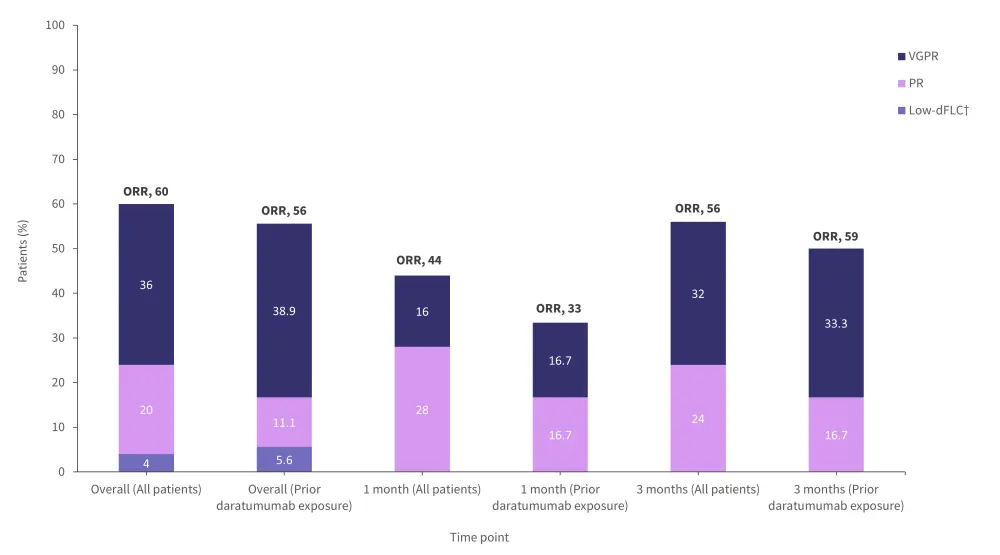
dFLC, difference between involved free light-chain and uninvolved free light-chain; ORR, overall response rate; PR, partial response; VGPR, very good partial response.
*Adapted from Kastritis.3
†Patients experienced a response to treatment but had persistence of small, non-measurable amounts of monoclonal protein that could still affect outcomes.
- The median time to first hematologic response was 0.5 months (range, <0.1–4.9).
- The median time to first very good partial response was 1.4 months (0.5–3.5).
- The median duration of response was 5.8 months.
- The 3-month response rate for involvement of any organ was 20%, 12% for heart, and 8% for kidney.
Safety
- All patients experienced at least one AE.
- 32% experienced hematologic toxicity and 16% cardiac disorders.
- Cardiac disorders were unrelated to belantamab, and no new cardiac or renal toxicity signals were observed.
- Grade ≥3 TEAEs were experienced by 72% of patients.
- At least one SAE occurred in 32% of patients.
- Fatal SAEs were observed in 12% of patients.
- At least one ocular TEAE occurred in 96%.
- Grade 3 or 4 ocular TEAEs were recorded in 48% of patients.
- A breakdown of ocular AEs is shown in Figure 4.
Figure 4. Ocular AEs in patients treated with belantamab mafodotin*
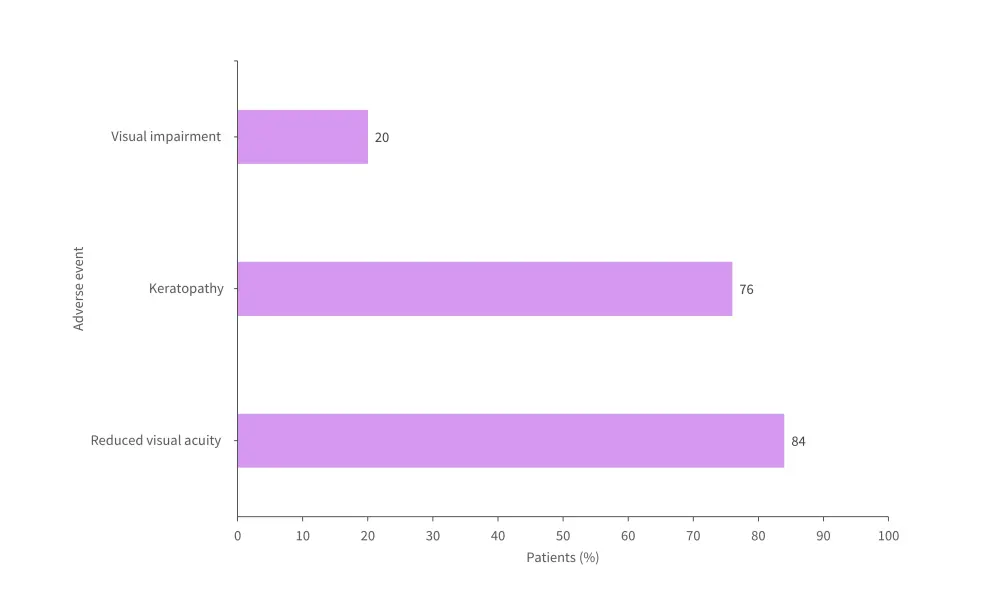
AE, adverse event.
*Adapted from Kastritis.3
Conclusion
The phase II trial of belantamab demonstrated a high hematologic response rate in heavily pre-treated patients with relapsed or refractory AL amyloidosis. The trial did not identify any new toxicity signals. Despite the prolonged administration period of belantamab, ocular toxicity could not be prevented. These results highlight a potential treatment option for patients with relapsed or refractory AL amyloidosis, particularly those with prior exposure to daratumumab and bortezomib.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?

