All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Life after CAR-T: Clinical outcomes of patients with MM who relapse after BCMA-targeted CAR T-cell therapy
Your opinion matters
How would you treat a patient who has relapsed after BCMA-targeted CAR T-cell therapy, assuming the patient is eligible for salvage therapy?
At the 2021 International Myeloma Workshop (IMW21) in September, Oliver Van Oekelen, from the Parekh Lab at the Icahn School of Medicine at Mount Sinai, presented data regarding the outcomes of patients with multiple myeloma (MM) who have relapsed following B-cell maturation antigen (BCMA)-targeted chimeric antigen receptor T-cell therapy (CAR-T),1 which we have summarized herein.
BCMA-targeting CAR-T and bispecific antibodies have changed the MM treatment landscape, offering hope to heavily pre-treated patients. While these therapies can produce deep responses, profoundly impacting the course of the disease, the responses are not always durable, with many patients eventually relapsing.1
Patient characteristics
What happens to these patients who relapse after CAR-T? Van Oekelen and colleagues looked at a cohort of 73 patients who were treated with BCMA-targeted CAR-T and identified 31 patients who relapsed following this treatment, starting their analysis at the time of relapse. The 31 patients in this cohort:
- had a median age of 61 years (range, 35‒75 years);
- had a median time from diagnosis of 74 months (range, 22‒282 months);
- had a median of five prior lines of treatment (range, 1‒18); and
- received one of three CAR-T products;
- most patients received either idecabtagene vicleucel (ide-cel) or ciltacabtagene autoleucel (cilta-cel), though several received BB21217.
The majority of the patients (84%) had high risk characteristics on fluorescence in situ hybridization (FISH). Table 1 summarizes the prior treatment exposure in this cohort.
Table 1. Prior treatment exposure*
|
ASCT, autologous stem cell transplantation. |
||
|
Treatment |
% Exposed |
% Refractory |
|---|---|---|
|
ASCT |
90 |
— |
|
Lenalidomide |
100 |
74 |
|
Pomalidomide |
87 |
84 |
|
Bortezomib |
90 |
61 |
|
Carfilzomib |
94 |
87 |
|
Ixazomib |
23 |
23 |
|
CD38 monoclonal antibody |
97 |
97 |
|
Alkylating agents |
100 |
54 |
|
Venetoclax |
19 |
19 |
|
Selinexor |
19 |
19 |
|
Bispecific antibodies |
13 |
13 |
|
Triple-class refractory |
— |
84 |
Post-CAR-T relapse
Of the 31 patients who relapsed after CAR-T, 28 received salvage therapy, with a median time from relapse to subsequent treatment of 30 days (range, 0‒201 days) and a median of two additional lines of treatment (range, 0‒8). The most common initial salvage regimen was chemotherapy-based; interestingly, five patients who relapsed after CAR-T were treated with a bispecific antibody on clinical trial as initial salvage therapy (Figure 1).
Figure 1. Salvage treatment schema*
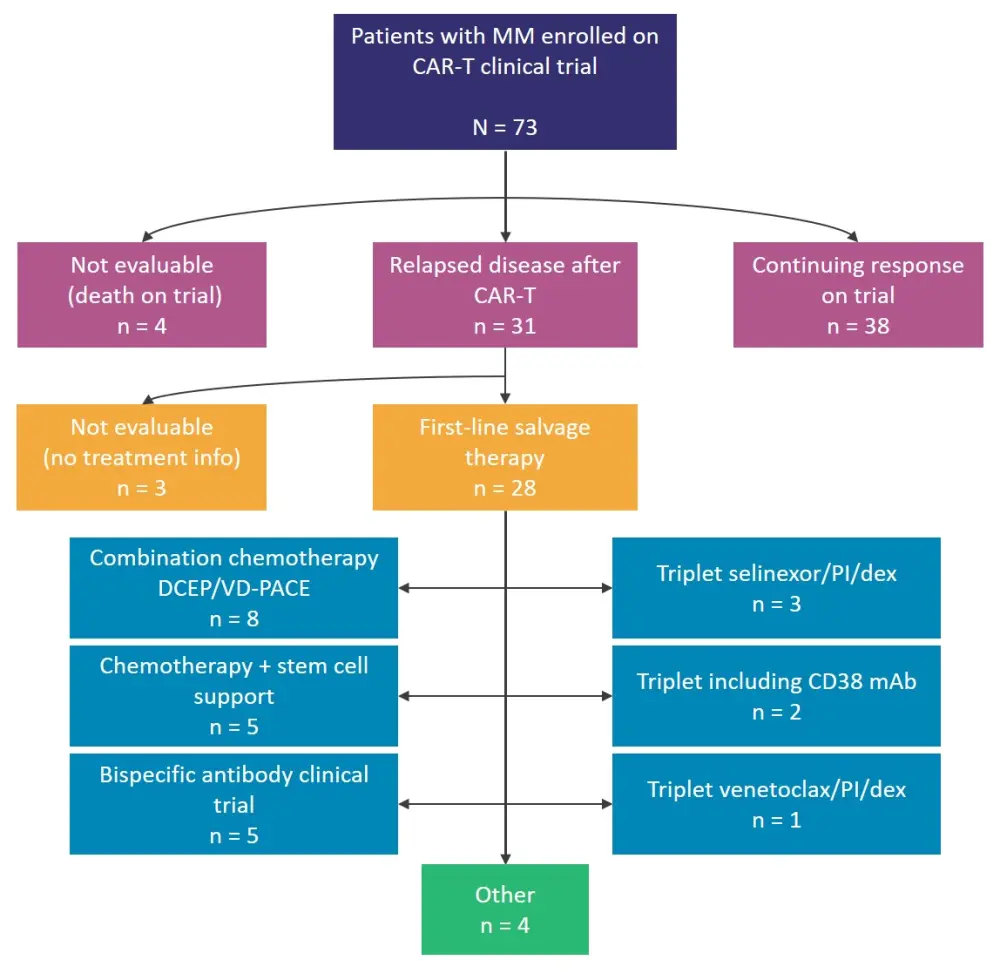
CAR-T, chimeric antigen receptor T-cell therapy; DCEP, dexamethasone, cyclophosphamide, etoposide, and cisplatin; dex, dexamethasone; mAb, monoclonal antibody; MM, multiple myeloma; PI, proteasome inhibitor; VD-PACE, bortezomib, dexamethasone, cisplatin, doxorubicin, cyclophosphamide, and etoposide.
*Adapted from Van Oekelen.1
The choice of treatment in the post-CAR-T relapse setting varies depending on patient characteristics: bispecific antibody therapy is an option for patients who are fit and clinical trial-eligible, while patients with cytopenias may benefit from stem cell support. Other options in this setting include triplet regimens containing selinexor, venetoclax, or CD38-targeting monoclonal antibody therapy.
Outcomes after relapse
- The overall response rate for the initial salvage therapy was 46%, with widely varying depths of response: seven patients had a complete response, five patients had a very good partial response, seven patients had stable disease, and eight patients had progressive disease.
- Of note, responses to bispecifics after CAR-T were often durable and, in some cases, are ongoing.
- The median progression-free survival for first line salvage treatment was 105 days (95% confidence interval, 78‒204 days).
- The median overall survival was 455 days, with a median follow-up of 501 days.
Looking at durable responses after relapse of BCMA-targeted CAR-T, with an arbitrary cutoff of 120 days, there were 33 occurrences of responses >120 days (range, 128‒555 days) at various treatment lines post-relapse. Treatment regimens inducing durable response included:
- chemotherapy + stem cell support (n = 8);
- bispecific antibodies, including BCMA-targeted (n = 8);
- selinexor + doublet (n = 5); and
- mitogen-activated protein kinase inhibition +/− other (n = 3).
Further characterization of CAR-T relapse
The collection of peripheral blood mononuclear cells and bone marrow aspirates at screening, various timepoints post-infusion, and relapse will be useful in characterizing relapse, as will the elucidation of factors contributing to relapse, including:
- clinical characteristics;
- tumor genomics;
- tumor microenvironment;
- cytokine milieu; and
- antigen loss.
Conclusion
The approval (and continued development) of BCMA-targeted CAR-T for the treatment of MM has changed the treatment landscape, as CAR-T has had a profound impact on disease course. While many patients still relapse following CAR-T, there are options for these patients, with an overall response rate of 46% and progression-free survival of 105 days in the first-line salvage setting and, in some cases, multiple lines of salvage treatment have contributed to an overall survival of 15 months. Further study is needed in this area to determine the factors contributing to relapse and to determine the impact of prior lines of therapy on response to treatment (including repeat therapies) in the post-CAR-T salvage setting.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?

