All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Isatuximab + VRd in auto-HSCT ineligible NDMM: Phase IB trial
Patients with multiple myeloma (MM) who are not eligible for autologous hematopoietic stem cell transplantation (auto-HSCT) have poor survival outcomes, despite advances in treatment. This is mainly attributed to patients receiving low intensity therapy due to their age and/or comorbidities.
Based on the phase III SWOG S0777 trial (NCT00644228), bortezomib-lenalidomide-dexamethasone (VRd) has become the standard-of-care regimen in patients with newly diagnosed MM (NDMM) who are ineligible for auto-HSCT.1 The addition of monoclonal antibodies, such as daratumumab, have shown decreased risk of disease progression.1
Isatuximab is an immunoglobulin G1 monoclonal antibody that targets a specific epitope of CD38; this has been extensively covered by the Multiple Myeloma Hub. Isatuximab has been approved in combination with carfilzomib and dexamethasone for the treatment of relapsed/refractory MM. Here, we summarize a recently published phase I trial by Ocio et al. in Leukemia evaluating isatuximab plus VRd in patients with NDMM who are not eligible for auto-HSCT.1
Study design
This is a phase I, open-label, multicenter trial (NCT02513186) in patients with NDMM ineligible for, or without the intent for transplantation. Study design is shown in Figure 1.
Figure 1. Study design*
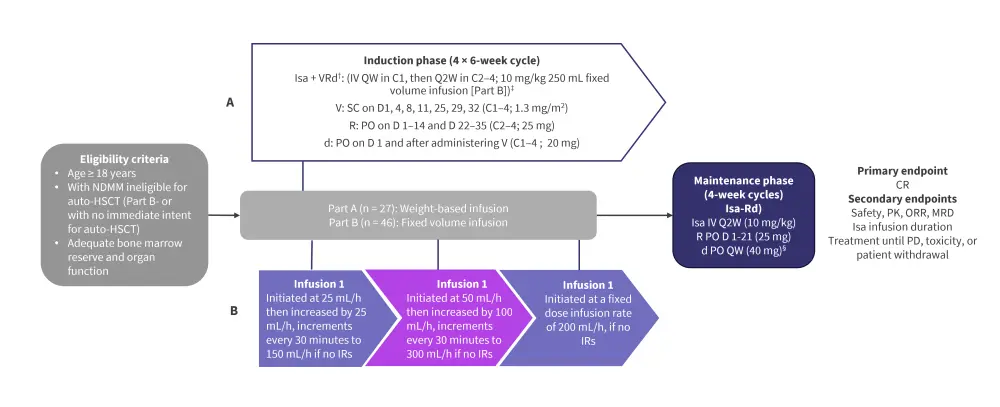
Auto-HSCT, autologous hematopoietic stem cell transplantation; C, cycle; CR, complete response; d, dexamethasone; D, day; IR, infusion reaction; Isa, isatuximab; IV, intravenously; MRD, minimal residual disease; NDMM, newly diagnosed multiple myeloma; ORR, overall response rate; PD, progressive disease; PK, pharmacokinetics; PO, orally; QW, once weekly; Q2W, every other week; R, lenalidomide; SC, subcutaneous; V, bortezomib.
*Adapted from Ocio et al.1
†Pre-medications included diphenhydramine 25–50 mg IV (or equivalent), dexamethasone 20 mg IV/PO, H2 antagonists, acetaminophen 650–1000 mg PO, montelukast 10 mg PO.
‡Isa 10 mg/kg diluted and administered IV from a fixed-volume infusion bag containing 250 mL of 0.9% sodium chloride solution.
§20 mg/day in patients >75 years old.
Results
A total of 73 patients were included; 27 in part A and 46 in part B.
- The median age was 71 years (range, 49–87 years), with 20.5% of patients aged ≥75 years
- Around 91.8% of patients were graded as International Staging System Stage I or II
- 20.4% of patients had high-risk cytogenetics
- 30.4% presented with 1q21+ abnormalities
Infusion duration
- The median duration of the first infusion was 3 hours 44 minutes and 3 hours 25 minutes in patients receiving weight-based and fixed-volume infusion, respectively, in part A.
- The median duration decreased to 2 hours 49 minutes and 1 hour 48 minutes for the second infusion.
- The median duration decreased from 3 hours 41 minutes for first infusion to 1 hour 55 minutes for second infusion in part B.
Efficacy
- Among 71 patients included in the efficacy analysis, the overall response rate was 98.5% (Figure 2).
- Overall response rate was similar among patient subgroups, including those with high-risk cytogenetics, older patients, and those with renal impairment.
- Of note, two patients with renal impairment at baseline achieved a complete response during treatment.
Figure 2. Best overall response*
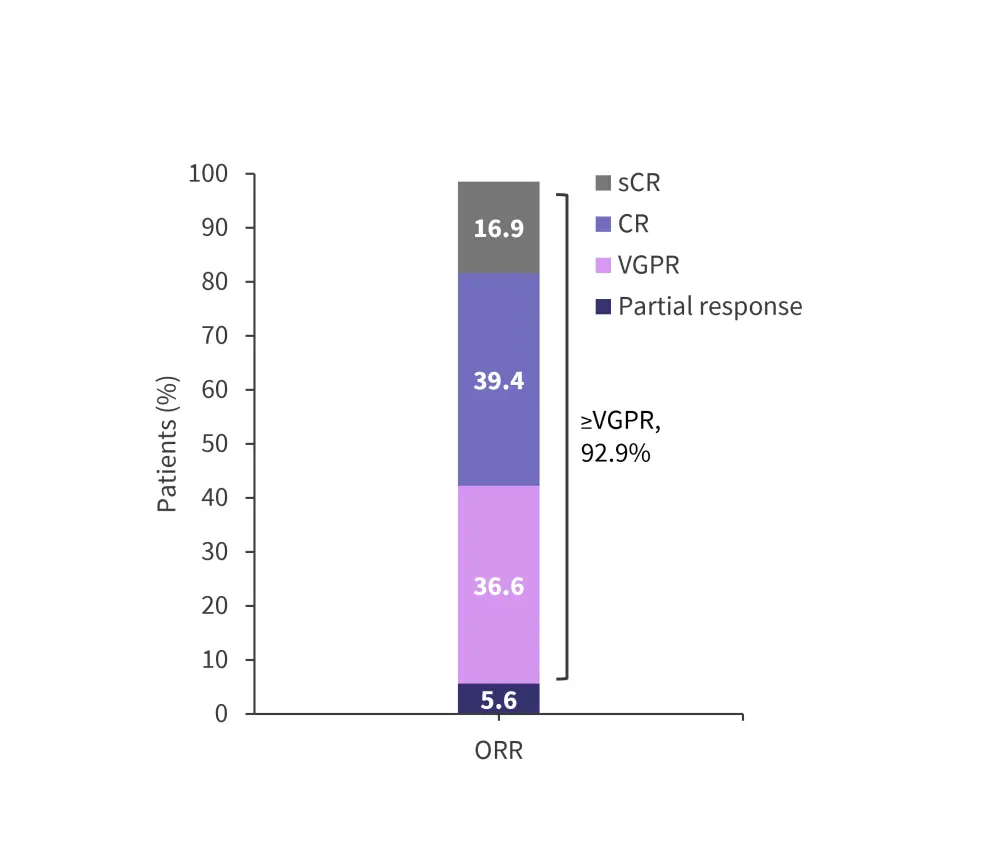
CR, complete response; ORR, overall response rate; sCR, stringent complete response; VGPR, very good partial response.
*Adapted from Ocio, et al.1
Minimal residual disease negative status (sensitivity 10−5) and (sensitivity 10−6) was achieved by 50.7% and 42.3% of patients, respectively. Minimal residual disease negative status based on best overall response is shown in Figure 3.
Figure 3. MRD negative by best overall response*
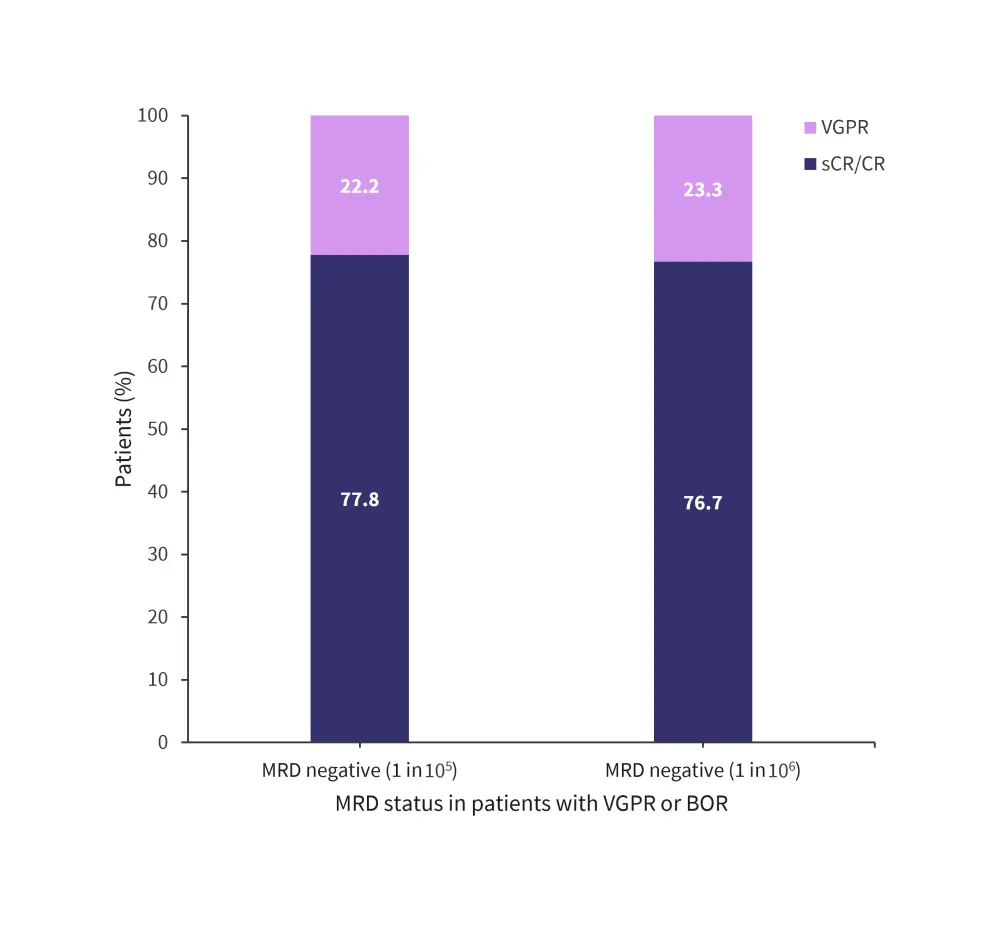
BOR, best overall response; CR, complete response; sCR, stringent complete response; MRD, minimal residual disease; VGPR, very good partial response.
*Adapted from Ocio, et al.1
- At a median follow-up of 35.8 months, progression-free survival (PFS) occurred in 6 of 26 patients in part A and, at a median follow-up of 25.3 months, PFS occurred in 10 of 45 patients in part B.
- Median PFS was not reached for the entire cohort, and the median probability of achieving PFS was 91% and 83.1% at 1 and 2 years, respectively.
- In part A, at a median follow-up of 37.9 months, overall survival occurred in 2 of 26 patients, while in part B, overall survival occurred in 7 of 45 patients at a median follow-up of 26.2 months.
Safety
- A total of 73 patients were included in the safety analysis. Any grade treatment and Grade ≥3 treatment-emergent adverse events (TEAEs) occurred in 100% and 79.5% of patients, respectively (Table 1).
- The most common TEAEs occurring in ≥20% of patients included constipation (68.5%), diarrhea (64.4%), asthenia (63%), peripheral sensory neuropathy (61.6%), peripheral edema (46.6%), and infusion reactions (41.1%).
- Any Grade infections were observed in 82.2% of patients, and Grade ≥3 infections in 23.3% of patients.
- First infusion reactions occurred in 73.3% of patients, with most being Grade 2.
- Only one Grade ≥3 infusion reaction occurred in part A, resulting in discontinuation.
- Grade 3–4 hematologic abnormalities in part A and B are shown in Figure 4.
Table 1. TEAEs*
|
TEAEs, treatment-emergent adverse event; SAEs, serious adverse events. |
|
|
TEAEs, % |
Total (N = 73) |
|---|---|
|
Any TEAEs |
100 |
|
Grade ≥3 TEAEs |
79.5 |
|
Treatment-emergent SAEs |
53.4 |
|
TEAEs leading to death† |
9.6 |
|
TEAEs leading to study dose reduction |
95.9 |
|
TEAEs leading to permanent discontinuation of study treatment |
19.2 |
|
TEAEs leading to premature study drug discontinuation |
32.9 |
|
Bortezomib |
17.8 |
|
Lenalidomide |
15.1 |
|
Dexamethasone |
5.5 |
Figure 4. Grade 3–4 hematologic abnormalities in part A and B*
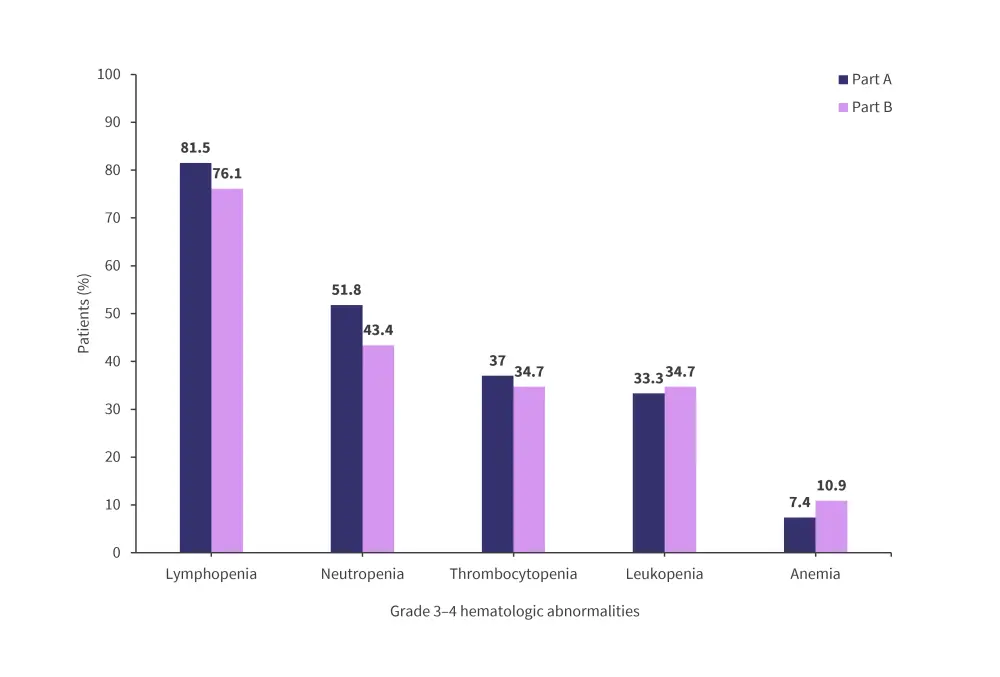
*Adapted from Ocio, et al.1
Pharmacokinetics
Isatuximab when combined with VRd showed that the area under the plasma concentration versus time curve from 0 to 1 week were within the previously reported range.
Conclusion
This phase I trial demonstrated the efficacy and deeper responses with the quadruplet regimen isatuximab plus VRd in patients with NDMM who were ineligible/had no immediate intent of transplantation. The safety profile of the quadruplet regimen was also consistent with the safety profile of the individual drugs. Findings from this study will support additional studies of isatuximab in patients with NDMM, for example, the phase III IMROZ trial (NCT03319667) and the GMMG HD7 trial (NCT03617731) investigating isatuximab-VRd in transplant ineligible and eligible patients, respectively.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?


