All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Johnson & Johnson, Legend Biotech, Pfizer, Roche, and Sanofi. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
IMWG clinical practice guidelines for the treatment of multiple myeloma-related bone disease
After evaluating the existing literature and grading recommendations using the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) method, experts from the Bone Working Group (BWG) of the International Myeloma Working Group (IMWG) have issued updated clinical practice guidelines for the management of multiple myeloma-related bone disease. These were published in the February 2021 edition of Lancet Oncology.1
The grading approach evaluates recommendations according to the quality of available evidence, separating them into four different categories from A to D. Below, we summarize the highest quality of evidence recommendations (Grade A) provided for newly diagnosed and relapsed/refractory (R/R) multiple myeloma (MM); other recommendation grades, when mentioned, will be noted as such.
Bisphosphonates and denosumab
You can find a synopsis of the updated treatment recommendations by experts from the working group for myeloma-related bone disease in Table 1 below.
Table 1. Summary of revised Grade A recommendations for the treatment of myeloma-related bone disease*
|
MM, multiple myeloma; R/R, relapsed or refractory. |
|
|
Patient population |
Patients with newly diagnosed and R/R myeloma |
|---|---|
|
Choice |
First option |
|
Zoledronic acid (regardless of the presence of myeloma-related bone disease on imaging) for patients with newly diagnosed or R/R MM; also consider for patients at biochemical relapse |
|
|
Denosumab (only in the presence of myeloma-related bone disease on imaging; also consider for patients with renal impairment) |
|
|
Second option |
|
|
Pamidronic acid (when first-option agents are not available or contraindicated) |
|
|
Administration |
Zoledronic acid: 4 mg administered intravenously over 15-minute infusion |
|
Pamidronic acid: 30 mg or 90 mg administered intravenously over 45-minute (for 30 mg) or 2-hour (for 90 mg) infusion |
|
|
Denosumab: subcutaneous injection of 120 mg |
|
|
Duration and frequency |
Zoledronic acid |
|
Monthly during initial therapy and in patients with less than very good partial response (VGPR) |
|
|
If patients achieve a VGPR or better after receiving monthly administration for at least 12 months, the treating physician can consider decreasing the frequency of dosing to every 3 months or, on the basis of osteoporosis recommendations, to every 6 months or yearly, or discontinuing zoledronic acid |
|
|
If discontinued, it should be reinitiated at the time of biochemical relapse to reduce the risk of new bone event at clinical relapse |
|
|
Denosumab |
|
|
Continuously, monthly until unacceptable toxicity |
|
|
If discontinued, a single dose of zoledronic acid should be given to prevent rebound effects at least 6 months after the last dose of denosumab; also consider giving denosumab every 6 months |
|
|
Monitoring and preventive measures |
Creatinine clearance and serum electrolytes (monthly) for zoledronic acid, plus urinary albumin (monthly) for pamidronic acid should be monitored monthly, and dose adjustments should be made accordingly; these tests are not needed for denosumab |
|
Dental health (at baseline, then at least annually or if symptoms appear) for both bisphosphonates and denosumab |
|
|
Calcium and vitamin D supplementation is recommended for all patients for both bisphosphonates and denosumab, especially for those with renal impairment, but only after the normalization of serum calcium concentration in case of hypercalcemia |
|
|
Patient education for early recognition and reporting of adverse events for both bisphosphonates and denosumab |
|
Additional recommendations
- Zoledronic acid is preferred to clodronic acid due to its superiority in reducing skeletal-related events and in improving survival, particularly in patients with newly diagnosed MM and myeloma-related bone disease at diagnosis.
- Compared with placebo or no treatment, only zoledronic acid has shown both progression-free survival and overall survival benefits.
- Intravenous bisphosphonate administration is preferred over intravenous pamidronic acid or oral clodronic acid for outpatients.
- Denosumab is equivalent to zoledronic acid in delaying the time to first skeletal-related event after a MM diagnosis.
Other approaches: Cement augmentation, radiotherapy, and surgery
The BWG of the IMWG recommend balloon kyphoplasty and vertebroplasty (Grade C) for patients with painful vertebral compression fractures. Radiotherapy (Grade C) is suggested for uncontrolled pain, impeding or symptomatic spinal cord compression, or pathological fractures. The algorithmic approach used to guide the decision-making process of treating patients with neurologic signs and symptoms due to spinal cord compression is presented in Figure 1.
Figure 1. Recommendations for the use of cement augmentation, radiotherapy, and surgery in vertebral complications due to MM*
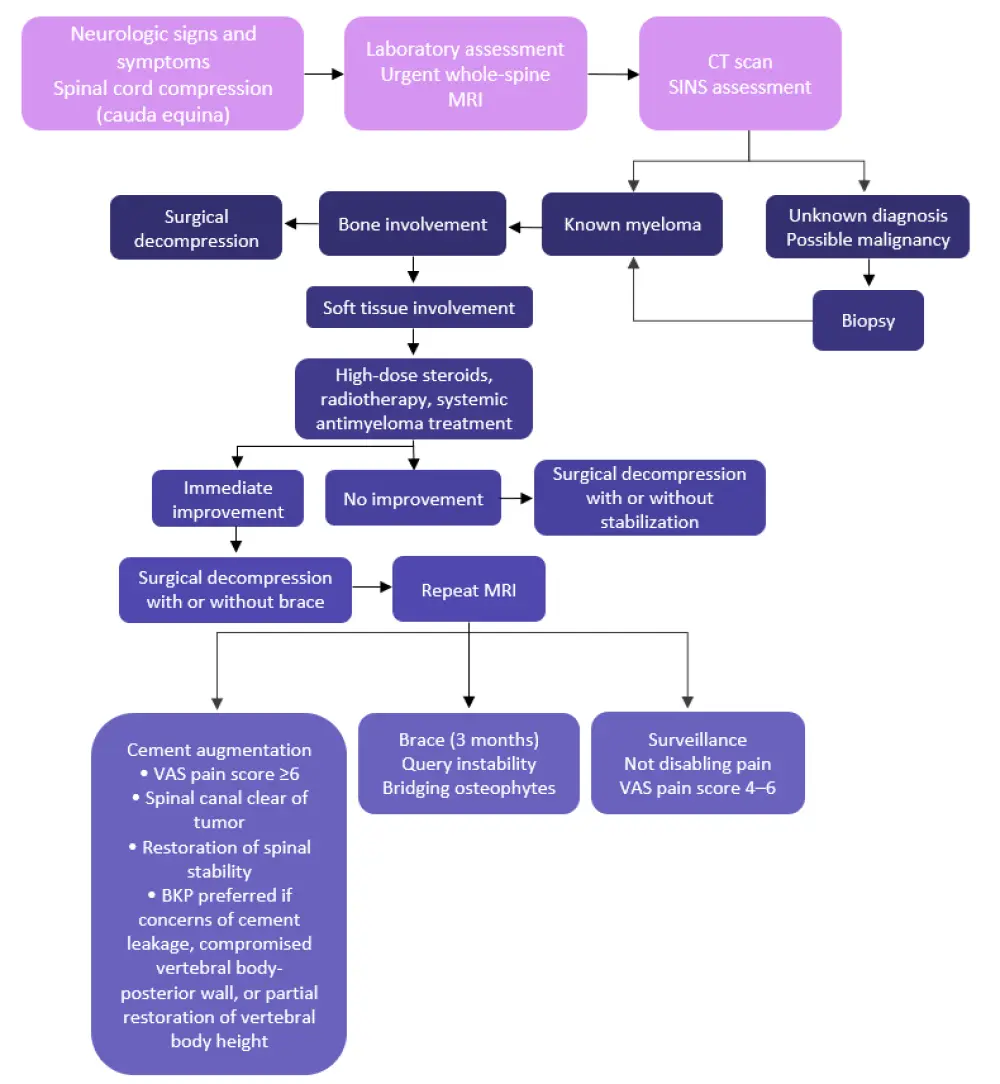
BKP, balloon kyphoplasty; CT, computed tomography; MRI, magnetic resonance imaging; SINS, spinal instability neoplastic score; VAS, visual analogue scale.
*Adapted from Terpos et al.1
Treatment considerations during the COVID-19 pandemic2
Table 2. Considerations for the prevention of skeletal-related events during the COVID-19 pandemic*
|
MM, multiple myeloma. |
|
|
Patient population |
Patients with symptomatic MM |
|---|---|
|
Choice |
First option |
|
Three monthly infusions of zoledronic acid |
|
|
Second option |
|
|
At home denosumab administration |
|
|
Administration |
For responding patients, subcutaneous administration of denosumab may be preferred over the intravenous infusion of bisphosphonates to reduce hospital visits or the length of hospital stay |
Summary
These recommendations aim to provide an optimal standard of care for the treatment of myeloma-related bone disease. The choice of bone-targeted agent, or any of the other approaches—cement augmentation, radiotherapy, and surgery—should be carefully considered according to the patients’ needs, presence of pathological fractures, convenience, and cost. Preventative measures are also of crucial importance to avoid treatment-related side effects.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

