All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Johnson & Johnson, Legend Biotech, Pfizer, Roche, and Sanofi. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Impact of ocular toxicities associated with belantamab mafodotin treatment in patients with RRMM
Do you know... In the pooled studies of DREAMM-7 and DREAMM-8 trials, in patients with RRMM treated with belantamab mafodotin, what was the median time to improvement of first bilateral reduction of BVCA in patients with normal baseline?
In the DREAMM-7 and DREAMM-8 phase III trials, belantamab mafodotin (belamaf) combined with standard therapies demonstrated statistically significant and clinically meaningful progression-free survival (PFS) benefit compared with standard-of-care regimens in patients with relapsed/refractory multiple myeloma (RRMM) after ≥1 prior line of therapy.1-3 While ocular events were common in both trials, they were effectively managed with dose modifications and were generally reversible with adequate follow-up, allowing patients to remain on treatment and experience clinical benefits.
Here, we present a visual abstract summarizing the ocular events associated with belamaf and the impact of dose modifications on safety and efficacy, as well as patient-reported outcomes.1–4
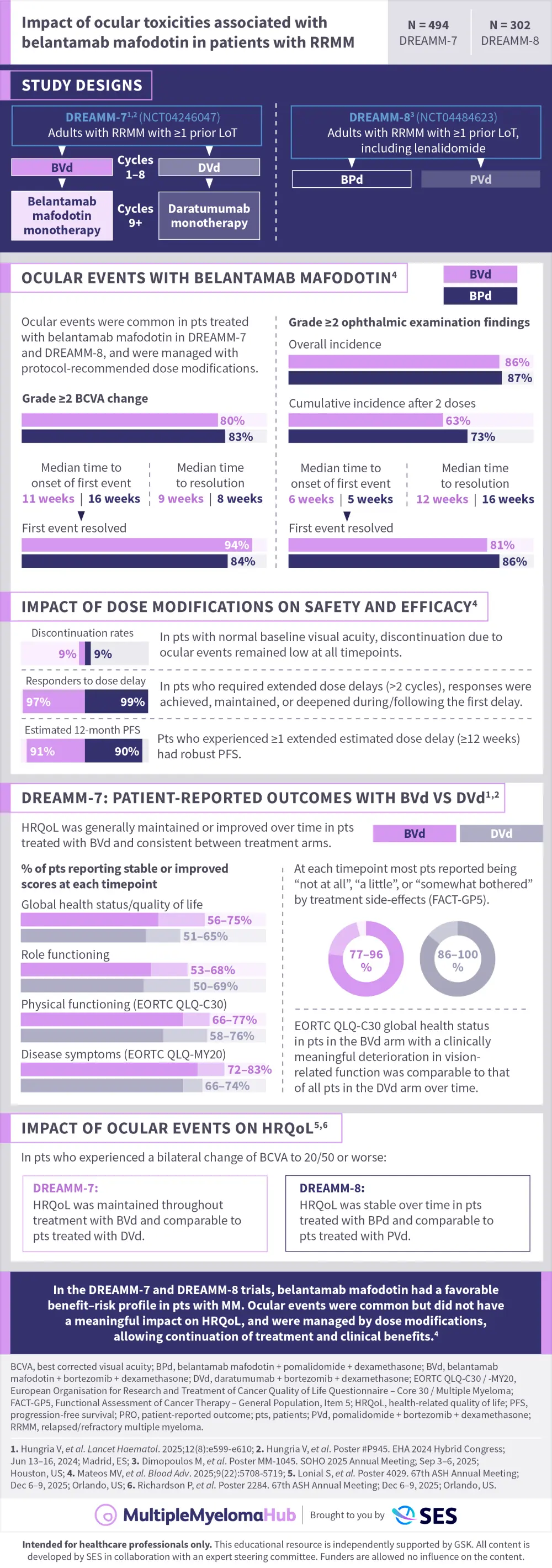
Key learnings:
Most patients experienced Grade 2 or 3 ocular events in both trials, which were managed with protocol-recommended dose modifications.3
Dose delays were common, with the median time between doses extending to every 8 and 12 weeks in DREAMM-7 and DREAMM-8, respectively.3
Despite dose modifications, efficacy was maintained; almost all patients who responded to treatment experienced ≥1 dose delay.3
In both studies, patients who required ≥1 extended dose delay of ≥12 weeks had robust PFS.
The majority of patients did not report having to stop driving or reading throughout treatment.3
In patients who experienced a bilateral change of BCVA decline to 20/50 or worse, health-related quality of life was stable over time in patients treated with BVd in the DREAMM-7 trial and BPd in the DREAMM-8 trial, and comparable with patients treated with DVd or PVd, respectively.5,6
Furthermore, EORTC QLQ-C30 global health status/quality of life, role of function, physical functioning, fatigue and pain were stable over time and consistent between BVd and DVd arms in the DREAMM-7 trial.1
This educational resource is independently supported by GSK. All content was developed by SES in collaboration with an expert steering committee; funders are allowed no influence on the content of this resource.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

