All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Impact of diabetes on MM survival outcomes across different racial groups
Do you know... Which of the following factors was associated with reduced overall survival among White but not Black patients with multiple myeloma?
Multiple myeloma (MM) is the most common hematologic malignancy affecting non-Hispanic Black adults, with an incidence rate twice that of White adults. The prevalence of diabetes mellitus is also higher in non-Hispanic Black (16.4%) compared with Hispanic (14.7%), non-Hispanic White (11.9%), and non-Hispanic Asian (14.9%) adults.1
Previous studies have correlated diabetes with an increased risk of MM, as well as increased mortality and reduced overall survival (OS) in patients with MM. Despite this, there are limited data on the prevalence and impact of diabetes on mortality in patients with MM across different racial groups, as well as the effects of diabetes on tumor growth in animal models.1
Here, we summarize a retrospective analysis by Shah et al.1 published in Blood Advances exploring racial differences in the prevalence and impact of diabetes on OS in patients with MM, as well as the effects of diabetes on in vivo tumor growth in mouse models.
Methods
This analysis included patients from two academic institutions in New York, Memorial Sloan Kettering Cancer Center and Icahn School of Medicine at Mount Sinai, who had MM with or without diabetes. The primary endpoint was OS, defined as the time from diagnosis until death or last follow-up.
For the in vivo and in vitro preclinical studies, a genetically engineered immunocompromised non-obese diabetic Rag1−/−/MKR mouse model was compared with a Rag1−/− control mice model.
Results
A total of 5,383 patients were included, with the cohort comprising patients who were predominantly White, male, >60 years of age, and had an elevated body mass index (BMI). Baseline characteristics are summarized in Table 1.
Table 1. Baseline characteristics*
|
BMI, body mass index. |
||||
|
Characteristic, % |
Patients with diabetes |
Patients without diabetes |
Total |
p value |
|---|---|---|---|---|
|
Race |
|
|
<0.0001† |
|
|
Black |
31.3 |
16.4 |
18.6 |
|
|
White |
68.7 |
83.6 |
81.4 |
|
|
Gender |
|
|
|
0.0486 |
|
Female |
41 |
44.8 |
44.2 |
|
|
Male |
59 |
55.2 |
55.8 |
|
|
Age |
|
|
|
<0.0001† |
|
<45 |
2.4 |
6.7 |
6.1 |
|
|
45−60 |
23.4 |
29.9 |
28.9 |
|
|
>60 |
74.2 |
63.4 |
65.0 |
|
|
BMI |
|
|
|
<0.0001† |
|
Underweight |
0.5 |
1.9 |
1.7 |
|
|
Normal |
19.2 |
31.8 |
29.9 |
|
|
Overweight |
32.4 |
39.7 |
38.6 |
|
|
Obese |
47.8 |
26.7 |
29.8 |
|
Prevalence of diabetes across racial groups by gender, age, and body mass index
Overall, there was a two-fold increase in the prevalence of diabetes in Black patients compared with White patients with MM (25% vs 12%); the prevalence was higher among Black patients across gender, age, and BMI group. Additionally, the prevalence of diabetes increased with higher BMI categories, higher age, and in male patients for both Black and White patients (Figure 1).
Figure 1. Prevalence of diabetes for Black and White patients with MM across gender, BMI, and age*†
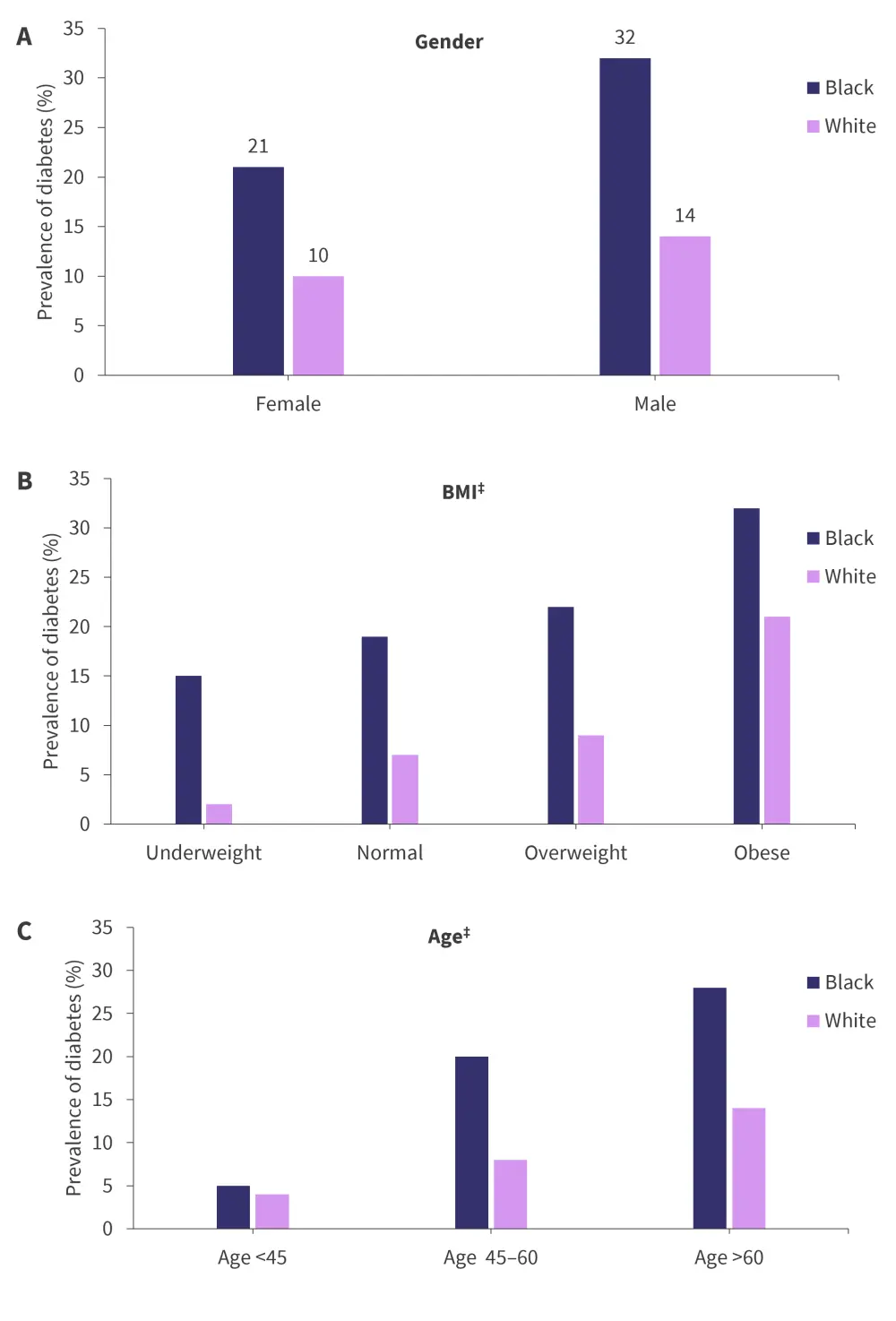
BMI, body mass index; MM, multiple myeloma.
*Data from Shah, et al.1
†p value was p < 0.0001 across gender, age, and BMI group.
‡Estimated values.
Impact of diabetes on overall survival
At a median follow-up of 4.62 years, univariate analyses revealed that patients with diabetes in the total cohort and White patients had a reduced OS compared with those without diabetes; however, there were no differences in OS in Black patients with and without diabetes (Table 2).
Table 2. OS by diabetes status and across racial groups in patients with MM*
|
CI, confidence interval; HR, hazard ratio; MM, multiple myeloma; OS, overall survival |
|||
|
Diabetes status |
Number of deaths/total |
HR (95% Cl) |
p value |
|---|---|---|---|
|
Total cohort |
|
<0.0001† |
|
|
Non-diabetic |
1,264/4,558 |
Reference |
|
|
Diabetic |
247/784 |
1.34 (1.17–1.54) |
|
|
Black cohort |
|
|
0.4785 |
|
Non-diabetic |
223/749 |
Reference |
|
|
Diabetic |
64/244 |
1.10 (0.83–1.45) |
|
|
White cohort |
|
|
<0.0001† |
|
Non-diabetic |
1,041/3,809 |
Reference |
|
|
Diabetic |
183/540 |
1.44 (1.23–1.69) |
|
In the pooled multivariate analyses, patients with diabetes in the total cohort had a significantly reduced OS compared with those without diabetes (p < 0.001).
- Elevated BMI was associated with an improved OS vs normal weight.
- Obesity vs normal weight (hazard ratio [HR], 0.82; 95% confidence interval [CI], 0.71–0.93; p = 0.003).
- Overweight vs normal weight (HR, 0.83; 95% CI, 0.70–0.99; p = 0.035).
- Patients aged >60 years had a significantly reduced OS (p < 0.001) compared with those aged <45 years.
- Age was the only factor that was significantly associated with OS among both racial groups; however, diabetes, BMI, and gender had differing associations with OS among Black (Figure 2A) and White patients (Figure 2B).
Figure 2. Multivariable analysis of OS in A Black and B White patients*
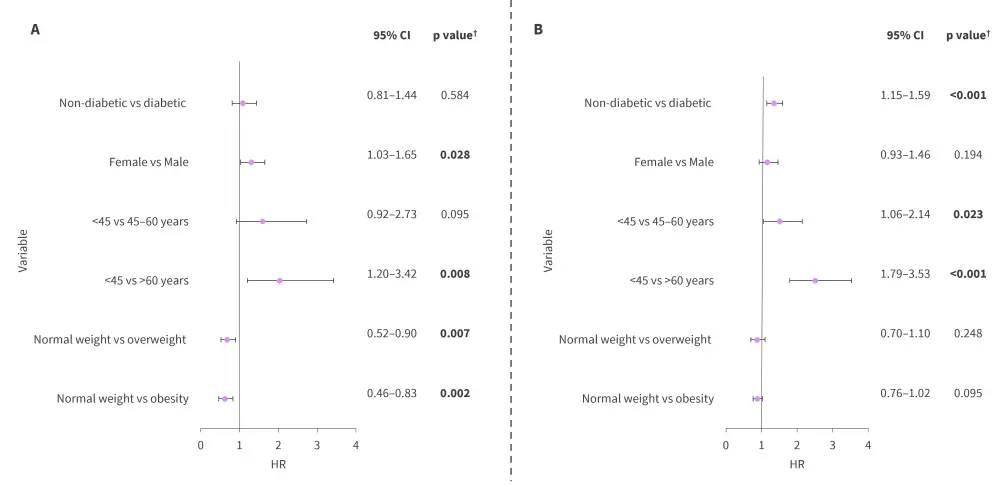
CI, confidence interval; HR, hazard ratio; OS, overall survival.
*Data from Shah, et al.1
†Values in bold are statistically significant.
Effect of diabetes on tumor growth in mouse models
Overall, MM.1S xenografts grew more rapidly in the Rag1–/–/MKR mice compared with the control mice. Western blot analysis showed that MM.1S xenografts had similar expression levels of the insulin receptor β protein between Rag1–/–/MKR mice and control mice. The analysis also revealed that MM.1S xenografts from Rag1–/–/MKR mice had higher phosphorylation of S6 ribosomal protein (Ser235/236) levels compared with controls, indicating greater activation of the mammalian target of rapamycin 70 pathway.
Conclusion
This study highlighted the higher prevalence of diabetes amongst Black patients compared with White patients with MM. Diabetes was associated with worse OS in the total cohort and among White patients, but not in Black patients. Diabetes also led to a faster progression of MM xenografts in mice models compared with those without diabetes. Other factors, such as age, elevated BMI, and gender had varying impacts on OS across Black and White populations. These findings suggest that risk factors, such as diabetes, should be managed to improve survival outcomes in MM.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?

