All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Identifying sensitivity to venetoclax: High response rates reported with VenDd in t(11;14) relapsed/refractory MM
Your opinion matters
Do you assess t(11;14) in your patients with myeloma? a) Not routinely b) Yes, at diagnosis c) Yes, at relapse d) Yes, at diagnosis and relapse
Venetoclax, an oral BCL-2 inhibitor, has been of particular interest in a specific subset of patients with t(11;14),1 a cytogenetic abnormality found in 15–20% of patients with multiple myeloma (MM). Venetoclax may enhance treatment response when combined with drugs that increase BCL-2 dependency, such as dexamethasone and bortezomib, and therapies that may eliminate resistant subclones to venetoclax, such as daratumumab.3
A phase I study sought to evaluate the safety and tolerability of venetoclax plus daratumumab and dexamethasone in patients with t(11;14) relapsed or refractory MM (RRMM), as well as the further addition of bortezomib in cytogenetically unselected patients. The results were published by Nizar Bahlis and colleagues in the Journal of Clinical Oncology2 and are summarized in this article.
Study design
Patients with RRMM aged ≥18 years were eligible if they had documented evidence of progression per the International Myeloma Working Group criteria during or after their last treatment regimen, an Eastern Cooperative Oncology Group performance status ≤2, acceptable laboratory parameters, and measurable disease confirmed by a central laboratory at screening.
The study was comprised of two parts (NCT03314181). In Part 1, venetoclax with daratumumab and dexamethasone (VenDd) was evaluated in patients with (t11;14) RRMM, and in Part 2, VenDd with bortezomib (VenDVd) was evaluated in cytogenetically unselected patients with RRMM. Patients in Part 1 must have received ≥1 prior line of therapy, including a proteasome inhibitor (PI) and immunomodulatory amide drug, and those in Part 2, must have received one to three prior lines of therapy and not be refractory to PIs.
Treatment schedule
VenDd treatment involved the following:
- VenDd treatment was administered in 28-day cycles.
- Venetoclax: 400 mg orally once daily, increased to 800 mg daily after determination of acceptable safety after Cycle 1 for additional patients enrolled.
- Daratumumab: 16 mg/kg initially administered intravenously (IV); however, the protocol was later amended to 1,800 mg subcutaneously weekly for the first two cycles, fortnightly for Cycles 3–6, then every 4 weeks thereafter.
- Dexamethasone: 40 mg administered weekly IV for the first dose, and IV or orally for subsequent doses. There was a 20 mg dose reduction for patients underweight or aged ≥75 years.
- Anti-infective prophylaxis and granulocyte colony-stimulating factor were recommended.
VenDVd treatment consisted of the following:
- VenDVd treatment was administered in 21-day cycles for Cycles 1–8, then in 28-day cycles.
- Venetoclax: Administered as for the VenDd cohort.
- Daratumumab: Given as for the VenDd cohort weekly for Cycles 1–3, every 3 weeks for Cycles 4–8, and every 4 weeks thereafter.
- Dexamethasone: 20 mg administered on Days 1, 2, 4, 5, 8, 9, 11, 12, and 15 of Cycles 1–3; Days 1, 2, 4, 5, 8, 9, 11, and 12 of Cycles 4–8; and 40 mg weekly thereafter.
- Bortezomib: 1.3 mg/m2 administered subcutaneously (preferred) or IV on Days 1, 4, 8, and 11 of Cycles 1–8.
- Antibiotic prophylaxis was mandated for patients receiving venetoclax with bortezomib.
Key results
A total of 48 patients were enrolled; 24 patients with t(11;14) RRMM in Part 1, and 24 patients with RRMM in Part 2. The median follow-up for Part 1 and Part 2 was approximately 21 months and 21.5 months, respectively. Baseline characteristics are summarized in Table 1. The differences between the Part 1 and Part 2 participants did not allow for direct comparison between the two study arms.
Table 1. Characteristics of patients in Part 1 (VenDd) and Part 2 (VenDVd)*
|
ECOG, Eastern Cooperative Oncology Group; IMiD, immunomodulatory imide drug; ISS, International Staging System; PI, proteasome inhibitor; VenDd, venetoclax + daratumumab + dexamethasone; VenDVd, venetoclax + daratumumab + bortezomib + dexamethasone. |
||
|
Characteristic |
Part 1 |
Part 2 |
|---|---|---|
|
Median age, years (range) |
63 (51–76) |
64 (41–80) |
|
ECOG performance status, n (%) |
|
|
|
0 |
13 (54) |
16 (67) |
|
1 |
11 (46) |
7 (29) |
|
2 |
0 (0) |
1 (4) |
|
ISS stage, n (%) |
|
|
|
I |
7 (29) |
9 (38) |
|
II and III |
14 (58) |
14 (58) |
|
Not evaluable or unknown |
3 (13) |
1 (4) |
|
Cytogenetic abnormalities, n (%) |
|
|
|
t(11;14) |
24 (100) |
6 (25) |
|
No. of prior lines of therapy, median (range) |
2.5 (1–8) |
1 (1–3) |
|
Prior PI, n (%) |
24 (100) |
22 (92) |
|
Refractory to prior PI |
11 (46) |
0 (0) |
|
Prior IMiD, n (%) |
24 (100) |
17 (71) |
|
Refractory to prior IMiD |
17 (71) |
8 (33) |
|
Prior PI plus IMiD, n (%) |
24 (100) |
15 (63) |
|
Refractory to prior plus IMiD |
10 (42) |
0 (0) |
Safety
Key safety outcomes are summarized in Table 2. The following notable points were observed:
- Nausea, diarrhea, and insomnia were common treatment-emergent adverse events for both treatment combinations.
- Fatigue was the most common treatment-emergent adverse event, with VenDd affecting 71% of patients.
- Grade ≥3 adverse events occurred in 88% of the VenDd cohort and 71% of the VenDVd cohort, the most common being hypertension and insomnia, respectively.
- There was one dose-limiting toxicity observed in Part 1 at 800 mg VenDd, which was Grade 3 febrile neutropenia.
- Infection occurred in 23 patients (96%) in the VenDd cohort and 15 patients (63%) in the VenDVd cohort, of which the most common was upper respiratory tract infection in both groups.
- One treatment-emergent death occurred in a patient in Part 2 (400 mg VenDVd) due to sepsis 3 weeks after treatment discontinuation because of disease progression and was not related to treatment.
Table 2. The most common any grade and Grade 3 and 4 TEAEs*
|
TEAE, treatment-emergent adverse event; VenDd, venetoclax + daratumumab + dexamethasone; VenDVd, venetoclax + daratumumab + bortezomib + dexamethasone. |
||||
|
TEAE |
Part 1 |
Part 2 |
||
|---|---|---|---|---|
|
Any grade |
Grade ≥3 |
Any grade |
Grade ≥3 |
|
|
Any TEAE, n (%) |
24 (100) |
21 (88) |
24 (100) |
17 (71) |
|
Nonhematologic TEAEs, n (%) |
|
|
|
|
|
Fatigue |
17 (71) |
2 (8) |
6 (25) |
1 (4) |
|
Diarrhea |
15 (63) |
2 (8) |
13 (54) |
2 (8) |
|
Nausea |
12 (50) |
1 (4) |
12 (50) |
0 (0) |
|
Insomnia |
10 (42) |
1 (4) |
13 (54) |
6 (25) |
|
Hypertension |
8 (33) |
4 (17) |
2 (8) |
0 (0) |
|
Peripheral sensory neuropathy |
0 (0) |
0 (0) |
6 (25) |
1 (4) |
|
Infection-related TEAEs, n (%) |
|
|
|
|
|
Any infection |
23 (96) |
6 (25) |
15 (63) |
5 (21) |
|
Upper respiratory tract infection |
9 (38) |
0 (0) |
5 (21) |
1 (4) |
|
Hematologic TEAEs, n (%) |
|
|
|
|
|
Neutropenia |
7 (29) |
5 (21) |
4 (17) |
1 (4) |
Efficacy
- An overall response rate of 96% was achieved with VenDd in patients with t(11;14) RRMM, and 92% with VenDVd in cytogenetically unselected patients.
- All responses in patients treated with VenDd were very good partial response or better, and 79% were very good partial response or better in those with VenDVd (Figure 1A).
- A reduction in serum M-protein levels of ≥50% was achieved in all patients.
- Minimal residual disease negative response (<10−5 by next-generation sequencing) was achieved in 33% of patients treated with VenDd (Figure 1B) and 21% treated with VenDVd (Figure 1C).
- In patients achieving complete response or better, 8 of 14 patients (57%) and 5 of 11 patients (45%) in Part 1 and Part 2, respectively, had minimal residual disease negativity, which was sustained for 6 months or more in 36% of participants in each group.
- In the VenDd cohort, which included heavily pretreated patients with t(11;14), 42% of which were refractory to both immunomodulatory amide drugs and PIs, the 18-month progression-free survival rate was 90.5.% (95% confidence interval [CI], 67.0–97.5).
- In the VenDVd cohort, the 18-month progression-free survival rate and the duration of response were 66.7% (95% CI, 42.4–82.5) and 70% (95% CI, 45.1–85.3), respectively.
Figure 1. Response rates and MRD negativity rates*
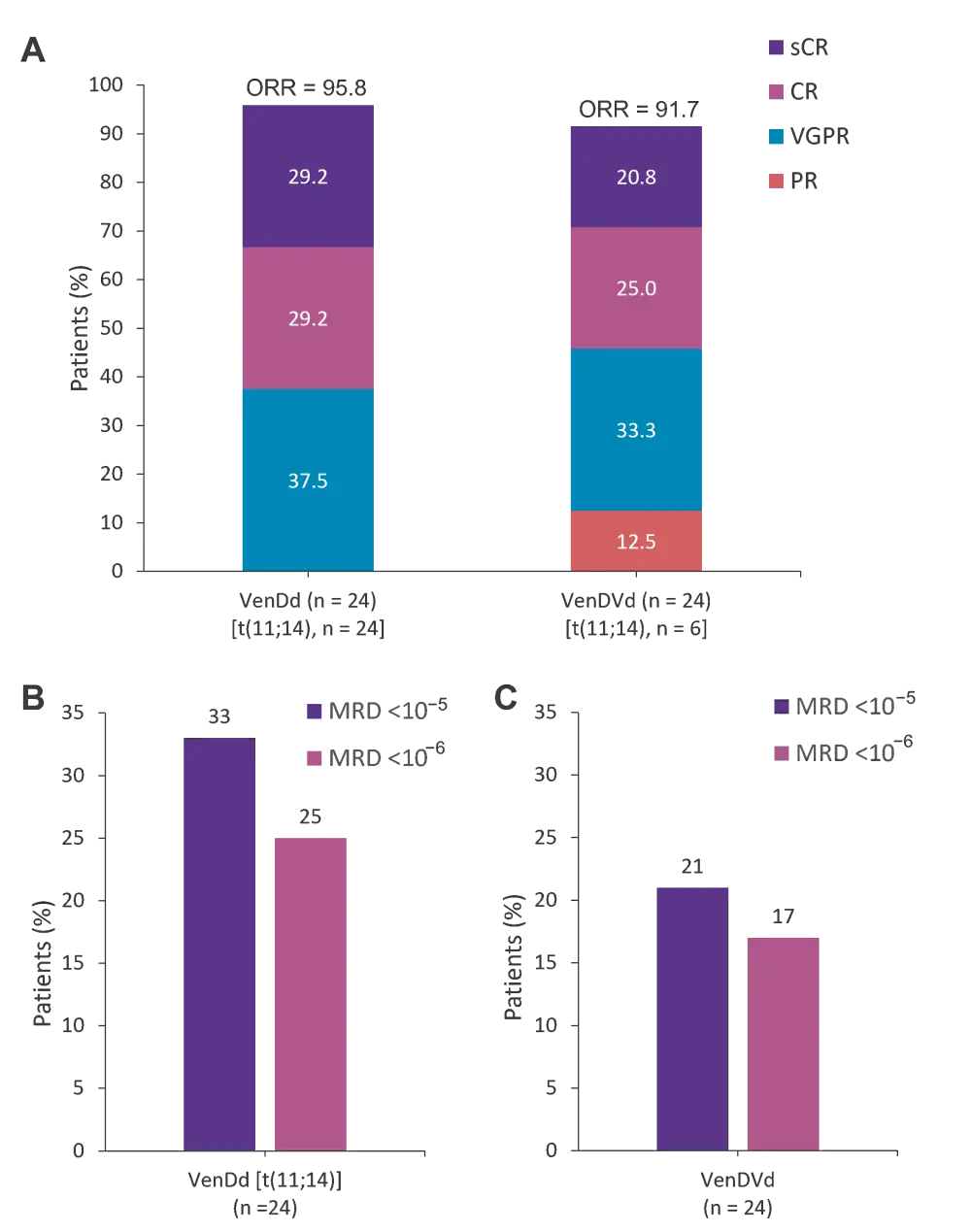
A Response rates and MRD negativity rates in patients treated with B VenDd in Part 1 and those treated with C VenDVd in Part 2. The 95% CIs for response rates in Part 1 were 78.9–99.9 for ORR, 36.6–77.9 for ≥CR, and 78.9–99.9 for ≥VGPR. The 95% CIs for response rates in Part 2 were 73.0–99.0 for ORR, 25.6–67.2 for ≥CR, and 57.8–92.9 for ≥VGPR.
CI, confidence interval; CR, complete response; MRD, minimal residual disease; ORR, overall response rate; PR, partial response; sCR, stringent complete response; VenDd, venetoclax, daratumumab, and dexamethasone; VenDVd, venetoclax, daratumumab, bortezomib, and dexamethasone; VGPR, very good partial response.
*Adapted from Bahlis, et al.2
Study conclusion
The authors concluded that both VenDd and VenDVd led to a high rate and sustained responses in patients with RRMM with and without t(11;14). Favorable responses with these new combinations versus venetoclax monotherapy,1 daratumumab monotherapy,3,4 and venetoclax and dexamethsone5 seen in previous studies suggest an additive effect when daratumumab is administered with venetoclax and warrants further investigation. Encouragingly, the safety profile was favorable, with no new safety signals identified. The phase II portion of the study will follow on after the high response rates in Part 1 with VenDd, enrolling patients with t(11;14) RRMM into a randomized, open-label cohort to further assess VenDd compared with daratumumab, bortezomib, and dexamethasone (DVd). On the other hand, in Part 2, the addition of bortezomib did not appear to be beneficial in patients identified as having t(11;14), and they experienced a higher rate of neuropathy. Therefore, Bahlis and colleagues conclude that the findings support further study of a personalized treatment approach, identifying predictive biomarkers to aid treatment decisions in BCL-2-dependent MM.
The future of venetoclax: Identifying additional markers of response to BCL-2 targeted treatment
Gupta and colleagues sought to better understand factors contributing to venetoclax sensitivity beyond t(11;14), and their findings were published in Blood.6 They studied 31 myeloma cell lines and 25 patient samples for venetoclax sensitivity and discovered venetoclax-sensitive myeloma retains a partially activated B‑cell gene expression program usually downregulated during plasma cell differentiation. They identified a B cell-like chromatin accessibility pattern suggestive of increased binding of a transcription factor involved in B‑cell development in venetoclax-sensitive cell lines. This process may drive BCL-2 dependence and therefore contribute to venetoclax sensitivity.
The aberrant B-cell pattern of gene expression described was observed almost exclusively in venetoclax-sensitive t(11;14) and not in venetoclax-resistant t(11;14), suggesting that t(11;14) alone could not explain this. No single gene was consistently expressed in venetoclax-sensitive samples; however, a panel of cell surface markers identified by flow cytometry correctly grouped MM cell lines and primary samples into sensitive and resistant to venetoclax. This panel included CD20, CD28, CD45, CD79a, and CD86. There is a possibility that a panel like this could be used to predict sensitivity to venetoclax and allow personalized treatment approaches for patients with MM beyond t(11;14).
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?

