All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Identifying and managing ocular toxicities in patients with MM
Do you know... Belantamab mafodotin (belamaf), an antibody-drug conjugate, was recently approved for the treatment of R/R MM. Several ocular toxicities requiring dose modifications have been reported. Identify one common ocular toxicity for belamaf.
Multiple myeloma (MM) is the second most prevalent hematologic cancer with a vast majority of patients requiring subsequent lines of therapy, leading to limited treatment options. Ocular toxicities are common adverse events (AEs) associated with molecularly targeted therapies and immunotherapies, as many of the essential signaling molecules that drive cancer growth are also expressed in ocular tissues.
An unprecedented case of transient myopic shift induced by daratumumab was reported in 2019 and the U.S. Food and Drug Administration (FDA) updated the drug label for daratumumab in 2021 to include ocular toxicity.1 With the advent of many novel therapies, the complexity of ocular pathology and understanding around ophthalmologic AEs occurring due to targeted therapies is important for the management of ocular toxicities in patients with MM.
During the 3rd European Myeloma Network Meeting, Marc Labetoulle discussed the early recognition and management of ocular toxicity to improve patient outcomes.2 Here, we present a summary of the ocular toxicities associated MM including recommendations for early detection and clinical management.
Ocular AEs associated with MM therapies
There are a range of ocular toxicities (Table 1) associated with both conventional chemotherapeutic agents as well as more recent agents such as belantamab mafodotin (belamaf), an antibody-drug conjugate (ADC) approved by FDA in 2020 for the treatment of relapsed and refractory MM. Figure 1 shows how specific MM drugs lead to ocular toxicities affecting different parts of the eye.
Table 1. Ocular AEs with MM therapies*
|
ADC, antibody-drug conjugate, AE, adverse event; BCMA, B cell maturation antigen; CD, cluster of differentiation; NOS, not otherwise specified; MM, multiple myeloma. |
||
|
Drug class |
Main side effects |
Others |
|---|---|---|
|
Alkylating agent |
||
|
Cyclophosphamide |
Blurred vision Visual impairment |
Conjunctivitis Eye oedema† Lacrimation increased |
|
Carmustine |
Ocular toxicities Transient conjunctival flushing and blurred vision Retinal hemorrhages |
Neuroretinitis |
|
Proteasome inhibitor |
||
|
Bortezomib |
Eye swelling‡ Vision abnormal‡ Conjunctivitis‡ Eye hemorrhage‡ Eyelid infection‡ Eye inflammation‡ Chalazion‡ Blepharitis‡ Diplopia Dry eye‡ Eye irritation‡ Eye pain Lacrimation increased Eye discharge |
Corneal lesion‡ Exophthalmos Retinitis Scotoma Eye disorder (including eyelid) NOS Dacryoadenitis acquired Photophobia Photopsia Optic neuropathy§ Different degrees of visual impairment (up to blindness) §
|
|
BCMA-targeted ADC |
||
|
Belantamab mafodotin |
Keratopathy‖ Microcyst-like epithelial changes Changes in visual acuity Blurred vision events¶ Dry eye events# Photophobia Eye irritation |
Ulcerative keratitis Infective keratitis |
|
MEDI2228 |
Photophobia |
|
|
CD138-targeted ADC |
||
|
Indatuximab ravtansine (BT062) |
Dry eye Lacrimation increased Vision blurred |
Angle closure Cataract Cystoid macular edema |
Figure 1. Ocular toxicities associated with MM therapies and anatomy of the eye*
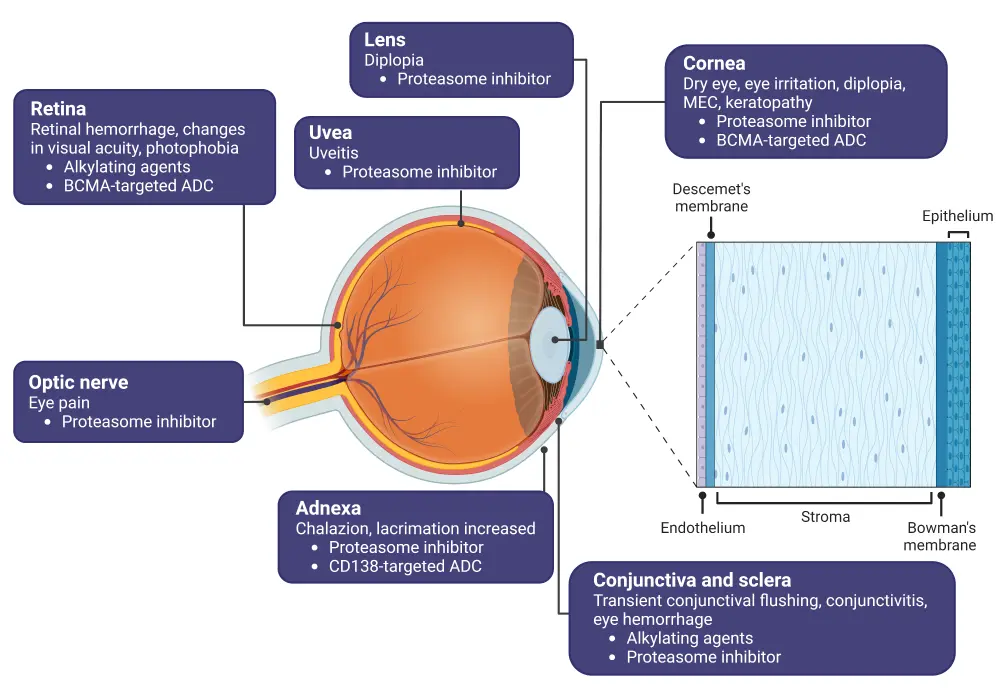
ADC, antibody-drug conjugate; BCMA, B-cell maturation agent; MEC, microcyst-like epithelial change; MM, multiple myeloma.
*Created with BioRender.com.
Mechanism of action
ADCs can reach the cornea either through the tear film or the vascularized area of the limbus, and may cause ocular toxicity either via on- or off-target processes. On-target ocular toxicity is caused by non-cancerous cells expressing the target antigen, binding of the monoclonal antibody leads to the release of the cytotoxic drug (such monomethyl auristatin F and ravtansine). Off-target ocular toxicity results from proteases, esterases, and other blood enzymes inducing premature deconjugation of the drug, leading to endocytosis and pinocytosis; this exposes neighboring cells to cytotoxicity.
Belamaf
For belamaf, ocular toxicity may occur via macropinocytosis by corneal epithelial cells. Epithelial stem cells can internalize the ADCs, causing cytotoxicity, and their migration to the cornea leads to keratopathy and blurred vision. Some ADCs, such as trastuzumab, may also have an on-target mechanism and cause ocular lesions similar to belamaf.
Ocular toxicity associated with belamaf can be observed by slit lamp microscopy to identify superficial punctate keratopathy, microcyst-like epithelial changes and whorl-like keratopathy. The ocular lesions are diffuse and presented bilaterally, with patients reporting blurred or decreased vision and dry eye. Other tools used to measure structural changes in the corneal epithelium include:
- optical coherence tomography
- in-vivo confocal microscopy
Changes in the corneal epithelial thickness may cause refractive shifts (myopic or hyperopic) as well as changes in the corneal curvature. The DREAMM-2 trial (NCT03525678) showed that ocular AEs associated with belamaf were frequent, with keratopathy observed in >70% of patients; however, only 18% of patients showed best corrected visual acuity change to 20/50 or worse. In total, 3% of patients discontinued the study due to corneal events. Belamaf ocular toxicity is managed either by dose delays, dose reduction, or discontinuation. Table 2 shows the incidence of keratopathy and treatment changes in the DREAMM 1, 2, and 6 trials investigating belamaf.
Table 2. Treatment changes due to ocular toxicity in DREAMM (1, 2, and 6)*
|
NA, not applicable. |
|||
|
|
DREAMM-1 (NCT02064387) |
DREAMM-2 (NCT03525678) |
DREAMM-6 (NCT03544281) |
|---|---|---|---|
|
Incidence of keratopathy |
69 |
70 |
100 |
|
Median time to onset of keratopathy (range), days |
23 (1–84) |
36 (19–143) |
NA |
|
Median time to resolution of keratopathy (range), days |
35 (5–442) |
71 (57–99) |
NA |
|
Treatment gap |
49 |
47 |
83 |
|
Dose reduction |
46 |
23 |
39 |
|
Discontinuation of treatment |
2.9 |
1 |
0 |
Ocular AEs grading scale
Based on the corneal epithelial thickness and location of the cyst, the ocular AEs can be graded as shown in Table 3.
Table 3. Ocular AEs grading scale*
|
AE, adverse event; BCVA, best corrected visual acuity; MEC, microcyst-like epithelial change. |
||||
|
Ophthalmic assessment |
Changes in visual acuity from baseline |
Change in corneal epithelium |
Complementary objective criteria |
Recommendation |
|---|---|---|---|---|
|
No ocular AEs |
No change |
No |
No |
Continue treatment at current dose |
|
Mild ocular AE |
Decline of 1 line |
Mild superficial punctate keratopathy |
Density: non-confluent
Location: predominantly (≥80%) peripheral
Microcyst: few if any |
Continue treatment at current dose |
|
Moderate ocular AE |
Decline of 2 or 3 lines (and not worse than 20/200) |
Moderate superficial keratopathy with or without MECs, sub-epithelial haze (peripheral), or new peripheral stromal opacity |
Density: semi-confluent
Location: predominantly (≥80%) paracentral |
Withhold treatment until examination findings improve or BCVA reaches mild severity or better
Consider resuming treatment at a reduced dose of 1.9 mg/kg2 |
|
Severe ocular AE |
Decline > 3 lines |
Several superficial keratopathy involving central cornea Corneal epithelial defect |
Density: confluent
Location: predominantly (≥80%) central |
Withhold treatment until examination findings improve or BCVA reaches mild severity or better
For worsening symptoms consider treatment discontinuation |
Management
The following recommendations can be made for the clinical management and advise to patients to reduce the frequency of ocular AEs and increase the tolerance to the antimyeloma drugs.
- Use of keratopathy and visual acuity scale to decide future treatments of belamaf
- Recommend to patients not missing any planned ophthalmic examinations (at baseline, before subsequent three treatment cycles, and in case any symptoms occur during treatment)
- Prescribe preservative free lubricant eye drops to begin from Day 1 of Cycle 1 until the end of the treatment
- Advise using cooling eye mask during drug infusion
- Preservative free and low-grade steroid eye drops may be useful in short term pulses to relieve side effects
- Educate patients to declare any new ocular symptoms that may occur during treatment
- Advise patients to pay attention when driving or operating machines
- Avoid contact lenses until the end of the treatment
Conclusion
The ocular surface is prone to AEs during the treatment of patients with relapsed/refractory MM with ADC’s and, while these AEs are not severe, they are a common occurrence in many patients. The frequency of ocular AEs can be reduced by educating patients in the self-management of their symptoms and the early identification of any signs, educating physicians on the management of ocular toxicities, and planning regular check-up visits appropriately. In addition, drug developers and medical professionals should be aware of these possible ocular toxicities, facilitating early recognition and intervention in both preclinical and clinical settings.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?

