All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
First-in-human study of REGN5458, a novel BCMA bispecific antibody
The B-cell maturation antigen (BCMA) is the cornerstone of the current and future novel immunotherapies developed for multiple myeloma (MM). BCMA is expressed in normal and malignant plasma cells; thus, we can find a broad landscape of BCMA-directed therapies in development, including chimeric antigen receptor (CAR) T-cell therapies, bispecific antibodies, and antibody–drug conjugates.
The Multiple Myeloma Hub has previously covered preliminary data, presented at the 62nd American Society of Hematology (ASH) Annual Meeting and Exposition, of new bispecific antibodies and bispecific T-cell engagers in clinical development for patients with relapsed/refractory MM. Amongst them, the fully human BCMAxCD3 bispecific antibody, REGN5458, demonstrated an encouraging safety and efficacy profile with early and deep responses, along with low rates of cytokine release syndrome in previously treated triple-refractory patients (NCT03761108).
Preclinical studies with REGN5458 showed potent in vitro and in vivo anti-myeloma activity, similar to those observed with anti-BCMA CAR T cells. However, the investigators highlighted a more rapid cell death with the bispecific antibody.1
This article summarizes the updated safety, overall response, and response durability in patients treated in the phase I dose-escalation part of the trial, presented at the 63rd ASH Annual Meeting and Exposition by Jeffrey Zonder.2
REGN5458 phase I first-in-human study design
The phase I part of the trial aimed to ascertain the safety and tolerability, determine the dose-limiting toxicities, and establish a recommended phase II dose of REGN5458. The secondary objectives were to assess the duration of response (DOR) and minimal residual disease status, the evaluation of the pharmacokinetic profile, and the characterization of its immunogenicity. Responses were measured using the modified International Myeloma Working Group criteria.
Key selection criteria were:
- Patients with relapsed/refractory disease who have been treated with ≥3 prior lines of therapy, including a proteasome inhibitor, an immunomodulator, and an anti-CD38 antibody
- Patients considered triple-refractory should also be penta-exposed (two proteasome inhibitors, two immunomodulators, and one anti-CD38 antibody)
- Eastern Cooperative Oncology Group (ECOG) performance status ≤1
- No prior treatment with any BCMA-directed therapy, except antibody–drug conjugates
The data cut-off was June 2021; at this time, 68 patients were treated with REGN5458 in the dose-escalation cohort with full doses ranging from 3–800 mg, as shown in Figure 1.
Figure 1. REGN5458 phase I dosing schedule and dose escalation levels*
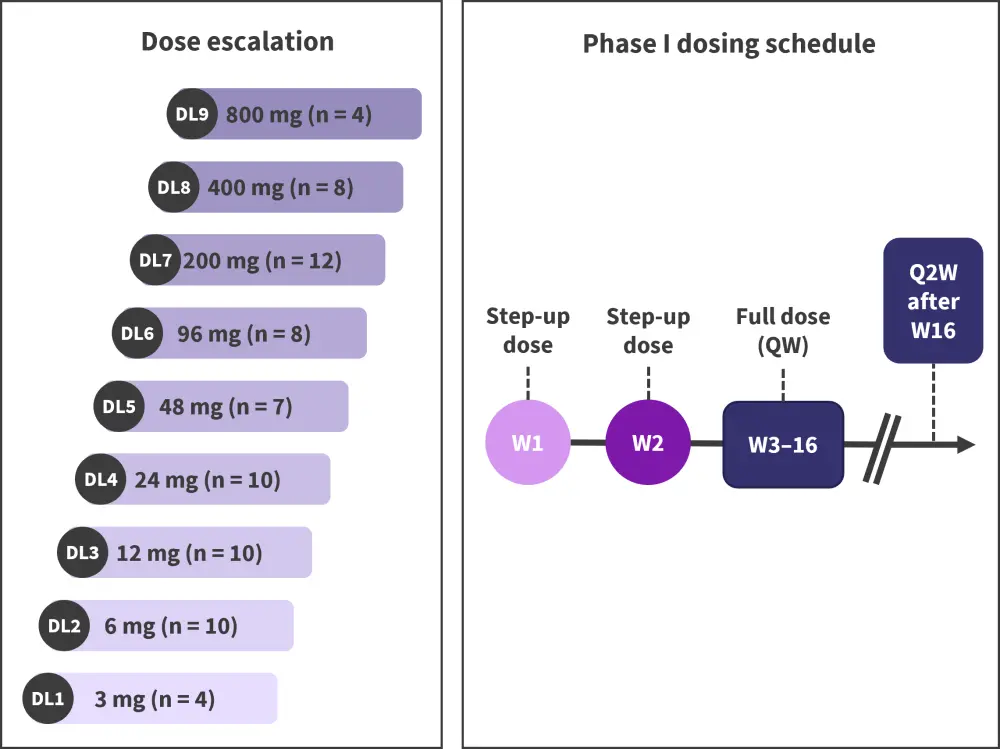
DL, dose level; QW, weekly; Q2W, every 2 weeks; W, Week.
*Adapted from Zonder.2
Initial efficacy and safety reported with REGN5458
Participating patients
- The median age at enrollment was 64 years (range, 41‒81 years; Table 2), and 21% of patients were ≥75 years.
- The median time to follow-up was 3 months (range, 0.7–22.1 months).
- Patients had a median of five previous lines of systemic therapy (range, 2–17), with 90% being refractory to the last line of treatment.
Table 2. Patient and disease characteristics*
|
BMPC, bone marrow plasma cell; ISS, International Staging System. |
|
|
Characteristic, % (unless otherwise stated) |
All patients (N = 73) |
|---|---|
|
Median age (range), years |
64 (41–81) |
|
Revised ISS stage at study entry |
|
|
I |
15 |
|
II |
58 |
|
III |
23 |
|
Prior autologous transplant |
64 |
|
Plasma cell burden (BMPC ≥50%) |
39 |
|
Cytogenetic high risk |
18 |
|
Refractoriness status† |
|
|
Triple-refractory |
19 |
|
Quad-refractory |
32 |
|
Penta-refractory |
38 |
Outcomes
- Responses were reported at all dose levels, with higher doses achieving higher response rates. The overall response rate among all enrolled patients was 51%.
- Dose-limiting toxicities were reported in two patients, one receiving 24 mg and the other 96 mg.
- Patients with extramedullary plasmacytomas responded less frequently than those without extramedullary plasmacytomas.
- Patients who received 200–800 mg had an overall response rate of 75%, and 58% had at least a very good partial response (VGPR).
- Amongst all responders, 86% achieved at least a VGPR, and 43% achieved at least a complete response (CR). Figure 2 displays the depth of response by dose interval.
- Four out of ten patients achieving a CR/stringent CR, were minimal residual disease negative at 10−5.
- The level of BCMA expression in the core biopsy did not correlate with disease response.
Figure 2. Best overall responses reported with REGN458*
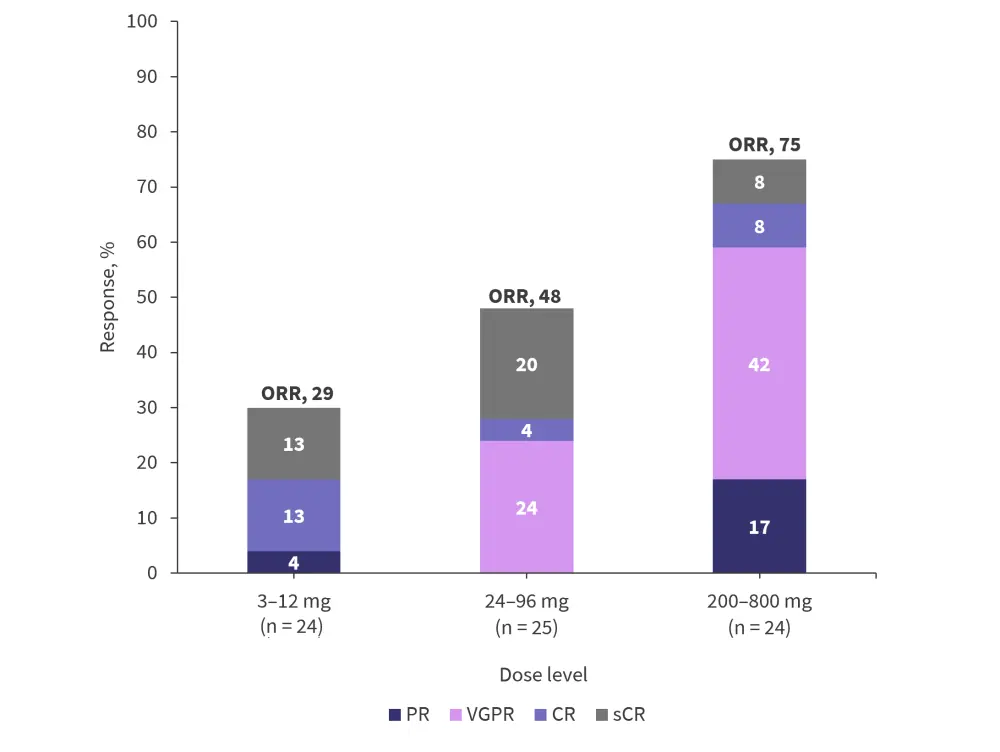
CR, complete response; ORR, overall response rate; PR, partial response; sCR, stringent CR; VGPR, very good partial response.
*Adapted from Zonder.2
Duration of response
- Responses occurred early, with 70% of responses occurring within the first 2 months.
- The median time to response was <1 month.
- The estimated median DOR was not reached.
- The probability of responders being event free at 8 months was 90.2% (95% confidence interval, 72.6–96.7), with patients still responding up to 19 months at the latest data cut-off.
Safety: Treatment-emergent adverse events
- Five deaths (7%) were reported in this study: three due to sepsis, one due to COVID-19, and one due to pneumonia.
- Treatment-emergent adverse events (TEAEs) of any grade were observed in 73% of patients, and Grade 3 or 4 TEAEs were seen in 42% and 33% of patients, respectively.
- Anemia of any grade was the most frequent hematologic TEAE (32%), followed by lymphopenia (23%) and neutropenia (23%).
- The most reported non-hematologic TEAEs of any grade were fatigue (45%), followed by cytokine release syndrome (CRS; 38%), pyrexia (36%), nausea (33%), and dyspnea (26%).
- CRS was mainly Grade 1, with only 4% patients experiencing Grade 2 CRS.
- CRS was most common during the first 2 weeks of REGN5458 dosing.
- No cases of Grade ≥3 CRS were reported, nor was there any discontinuation of treatment due to CRS. Table 3 provides further information on the CRS events.
- There was no relationship observed between CRS and dose level or CRS and response.
- No Grade 3 immune effector cell-associated neurotoxicity syndrome (ICANS) event was reported, and Grade 2 ICANS events were reported in 4% of patients.
Table 3. Cytokine release syndrome events*
|
CRS, cytokine release syndrome. |
|
|
Study population |
Total (N = 73) |
|---|---|
|
Patients with CRS of any grade, n (%) |
28 (38) |
|
Median time to first CRS onset (range), hours |
6–47 |
|
Median duration of CRS (range), hours |
14.7 (0–96) |
|
Patients receiving supportive measures to treat CRS, % |
|
|
Tocilizumab |
43 |
|
Steroids |
21 |
Conclusion
With the updated data of the first-in-human study with REGN5458, this novel BCMA-targeted bispecific antibody was deemed safe in patients with MM who were at least triple-refractory to previous therapies. At the dose level of 200–800 mg, the ORR was 75%, with 58% of patients achieving at least a VGPR. The results of this trial were encouraging and supported the continuation of the phase II portion of the study (currently recruiting) and the soon-to-be-initiated phase I trial that will explore REGN5458 in combination with currently approved MM therapies (NCT05137054).
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?

