All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Dermatological toxicities associated with talquetamab in RRMM
Talquetamab is a G protein-coupled receptor family C class 5 member D (GPRC5D) targeting bispecific antibody which was granted accelerated approval by the U.S. Food and Drug Administration and conditional marketing authorization by the European Commission. In both approvals, patients must have been treated with a proteasome inhibitor, an immunomodulatory agent, and an anti-CD38 antibody; making talquetamab the first GPRC5D-targeted bispecific antibody to be approved for use in relapsed/refractory multiple myeloma (RRMM).
Here, we summarize a retrospective study published by Lery et al.1 in Journal of the American Academy of Dermatology on the dermatological toxicities associated with the use of talquetamab for the treatment of RRMM.
Study design/patient population1
- A descriptive retrospective study conducted with patients treated in a single center from 2020–2022.
- The cohort consisted of 14 patients with RRMM who had been exposed to talquetamab.
- Eligibility criteria included both patients treated with talquetamab monotherapy or in combination.
- Doses of talquetamab included subcutaneous infusion between 300 μg/kg and 800 μg/kg, administered weekly or fortnightly.
Key findings1
- Of the 14 patients enrolled, 12 developed dermatological toxicities, with an overall incidence of 86% in this small cohort.
- Nail changes were the most commonly occurring presentation, observed in 79% of patients, with onychomadesis in 57% (Figure 1).
- The most frequent toxicity observed was xerostomia, occurring in 71% of patients.
- Most toxicities were Grade 1 or 2, with no patients discontinuing treatment due to toxicities.
- The dermatological toxicity profile remained consistent, regardless of the dose of talquetamab or combination administered.
- In all cases, an improvement in hand-foot syndrome, xerosis, and fissures was achieved with skin-directed therapies, including high-potency steroids and moisturizers.
Figure 1. Dermatological toxicities*
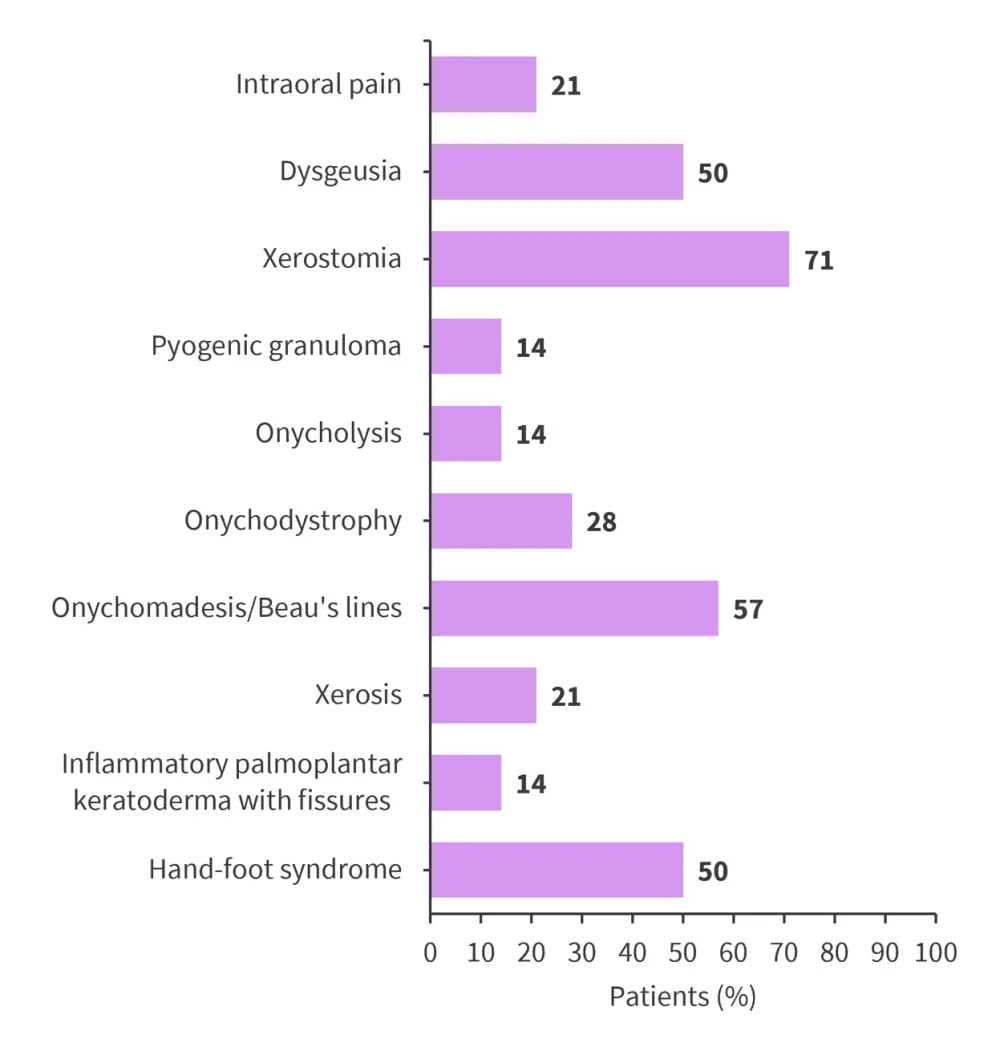
*Data from Lery, et al.1
|
Key learnings |
|---|
|
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?



