All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Changing practice patterns in autologous transplantation at EBMT centers
Over the last 25 years, the number of autologous hematopoietic cell transplantations (auto-HCT) for the treatment of transplant-eligible patients with multiple myeloma (MM) has increased.
During the Virtual 46th Annual Meeting of the European Society for Blood and Marrow Transplantation (EBMT), Patrick Hayden presented the results of a retrospective analysis of patients who underwent first auto-HCT for MM at EBMT centers between 1997 and 2018.1
Methods
This retrospective analysis evaluated the trends in transplant use, patient selection, induction regimen, response to transplant, choice of mobilization regimen, stem cell doses, and survival over consecutive 5-year cohorts:
- 1993–1997
- 1998–2002
- 2003–2007
- 2008–2012
- 2013–2017
All data were obtained from the EBMT registry.
Results
The study included 103,032 patients from 568 centers in 54 countries. Findings are reported in Table 1 and Figure 1.
Table 1. Study findings1
|
|
1993–1997 |
1998–2002 |
2003–2007 |
2008–2012 |
2013–2017 |
|---|---|---|---|---|---|
|
auto-HCT, autologous hematopoietic cell transplantation; CR, complete response; CTD, cyclophosphamide + thalidomide + dexamethasone; G-CSF, granulocyte colony-stimulating factor; PAD, bortezomib + doxorubicin + dexamethasone; PR, partial response; VCD, bortezomib + cyclophosphamide + dexamethasone; VD, bortezomib + dexamethasone; VRD, bortezomib + lenalidomide + dexamethasone; VTD, bortezomib + thalidomide + dexamethasone. *Data were available in 19,882 cases. |
|||||
|
No. of auto-HCT |
5,246 |
12,554 |
21,153 |
28,390 |
35,689 |
|
Median age at auto-HCT, years |
54 |
57 |
58 |
59 |
61 |
|
Patients over 65 years at auto-HCT, % |
3 |
9 |
14 |
14 |
22 |
|
Induction regimen, %* |
|
|
|
|
|
|
VTD |
— |
— |
— |
11 |
32 |
|
VCD |
— |
— |
— |
5 |
20 |
|
CTD |
— |
— |
— |
15 |
10 |
|
VD |
— |
— |
— |
19 |
7 |
|
PAD |
— |
— |
— |
5 |
4 |
|
VRD |
— |
— |
— |
2 |
3 |
|
Response rates post induction, % |
|
|
|
|
|
|
CR |
16 |
14 |
13 |
20 |
21 |
|
PR |
65 |
68 |
70 |
72 |
73 |
|
Stem cell mobilization regimens, %* |
|
|
|
|
|
|
Cyclophosphamide + G-CSF |
31 |
54 |
63 |
64 |
65 |
|
G-CSF only |
69 |
45 |
36 |
31 |
28 |
|
G-CSF + plerixafor |
— |
— |
— |
3.5 |
5 |
|
Stem cell doses, × 106 CD34+ cells/kg |
|
|
|
|
|
|
Total collection |
5.1 |
5.2 |
6.3 |
6.6 |
6.5 |
|
Dose infused |
3.6 |
4.1 |
4 |
3.8 |
3.8 |
|
Time from diagnosis to auto-HCT, months |
8.9 |
7.7 |
7.4 |
7.4 |
7.3 |
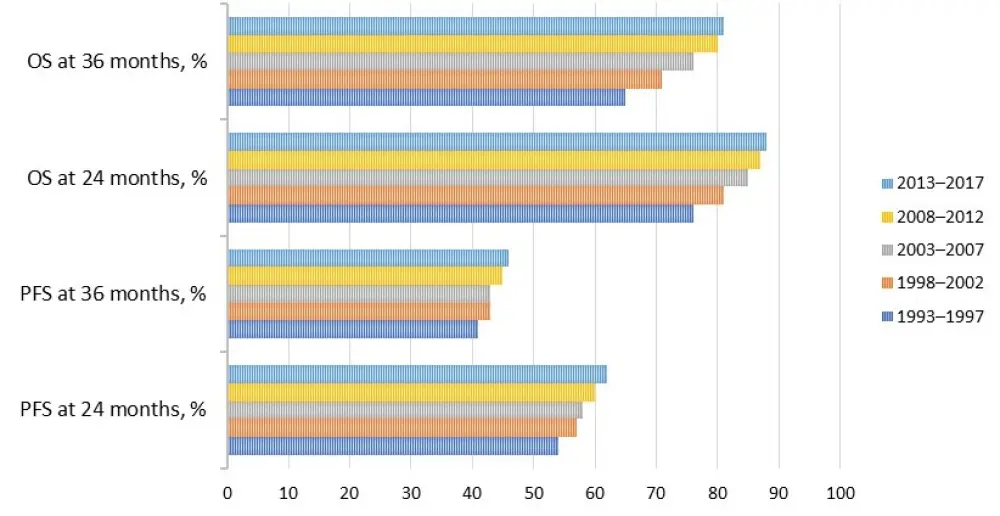
Figure 1. Progression-free survival and overall survival rates at 24 and 36 months1
Conclusion
Over the last 25 years, the number of transplants has increased sevenfold, and the median age at transplant and the percentage of patients > 65 years at transplant has also increased. The choice of induction regimens has evolved, with a growing number of patients having received bortezomib-based triplet induction in the 2013─2017 cohort. A rise in the number and quality of responses and a significant improvement in progression-free survival and overall survival rates were observed, which supports the critical role of auto-HCT even in the novel agent era.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?

