All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Cevostamab for relapsed/refractory multiple myeloma
Cevostamab targets the membrane-proximal domain of Fc receptor homolog 5 (FcRH5), which is expressed exclusively on B-lineage cells. Cevostamab, as an FcRH5/CD3 bispecific antibody, has shown promising activity in heavily pre-treated patients with relapsed/refractory multiple myeloma (RRMM). Cevostamab received orphan drug designation last year from both the U.S. Food and Drug Administration (FDA)1 and the European Medicines Agency (EMA)2.
During the 63rd American Society of Hematology Annual Meeting and Exposition, Suzanne Trudel3 presented the updated safety and efficacy data from a phase I study (NCT03275103) of cevostamab in patients with RRMM.
Study design
Inclusion criteria for the trial were as follows:
- Patients with RRMM who had exhausted other treatment options (prior CAR T-cell, antibody–drug conjugate, and bispecific antibodies allowed).
- An Eastern Cooperative Oncology Group performance score of 0–1.
Cevostamab was given intravenously for 17 cycles. To reduce cytokine release syndrome (CRS)/infusion reactions, Cycle 1 (C1) step-up dosing (Figure 1) was used, along with corticosteroid premedication in C1−2 and acetaminophen and diphenhydramine premedication in C1−17. Patients were hospitalized for ≥72 hours after each C1 infusion.
Figure 1. Cevostamab administration with single and double step-up dosing schemas*†
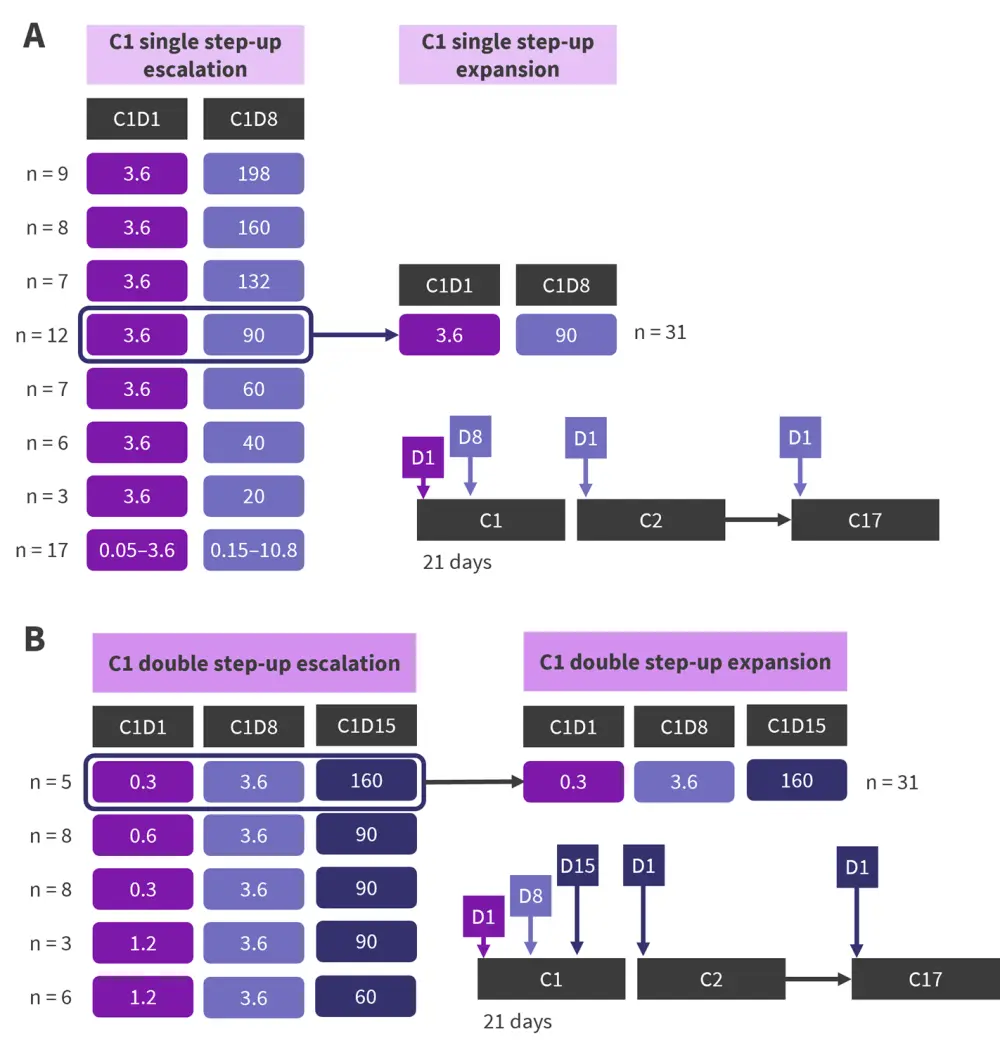
A Single step-up and B double step-up dosing schemas of cevostamab administration.
C, Cycle; D, Day.
*Adapted from Trudel, et al.3
†All doses in mg.
Results
The baseline characteristics of this heavily pretreated group of patients with RRMM are shown in Table 1. There was a high prevalence of patients with high-risk cytogenetics, with 70.5% having gain of 1q21. In total, 88.8% of patients were refractory to previous agents and had been treated with a median of six prior lines of therapy. Patients remained on the study for a median of 8.8 months (range, 0.2−37.2 months).
Table 1. Patient characteristics*
|
ADC, antibody drug conjugate; BCMA, B-cell maturation antigen; MM, multiple myeloma. |
|
|
Characteristic, % (unless otherwise stated) |
Total (N = 161) |
|---|---|
|
Median age (range), years |
64 (33−82) |
|
Male |
58.4 |
|
High-risk cytogenetics† |
|
|
1q21 gain |
70.5 |
|
t(4;14) |
55.6 |
|
t(14;16) |
13.5 |
|
del(17p) |
2.2 |
|
Extramedullary disease |
21.1 |
|
Median time since first MM therapy (range), years |
6.1 (0.3−22.8) |
|
Median number of prior lines of therapy (range) |
6 (2−18) |
|
Prior anti-CD38 antibody |
88.2 |
|
Prior anti-BCMA |
33.5 |
|
Prior ADC |
16.8 |
|
Prior bispecific antibody |
8.1 |
|
Triple-class refractory‡ |
84.5 |
|
Penta-drug refractory§ |
68.3 |
|
Refractory to previous therapy |
88.8 |
Safety
The most common Grade 3 and 4 adverse events (AEs) were:
- Neutropenia
- Anemia
- Thrombocytopenia
- Lymphopenia
- Infection
A total of 99.4% of patients experienced an AE during the study, with 32.9% at Grade 3 and 28.6% at Grade 4. Fatal AEs were recorded in 3.7% and one death was classified as cevostamab-related and recorded as due to hemophagocytic lymphohistiocytosis. Treatment discontinuation occurred in 13% of patients due to AEs, with 4.3% being related to cevostamab.
CRS
CRS was first seen in C1 and occurred within 24 hours of administration in 70% of patients. In 85% of patients, CRS then resolved within 48 hours. The profile of CRS and immune effector cell-associated neurotoxicity syndrome (ICANS) grades across the cohort is shown in Figure 2. Only one patient with ICANS + CRS did not have a resolution of their ICANS symptoms. This patient subsequently discontinued treatment due to disease progression. Most patients with CRS were managed with tocilizumab only (37.3%), while 21.7% had only steroids, and 20.0% were treated with both agents.
Figure 2. Patients who experienced CRS and ICANS after cevostamab infusion*
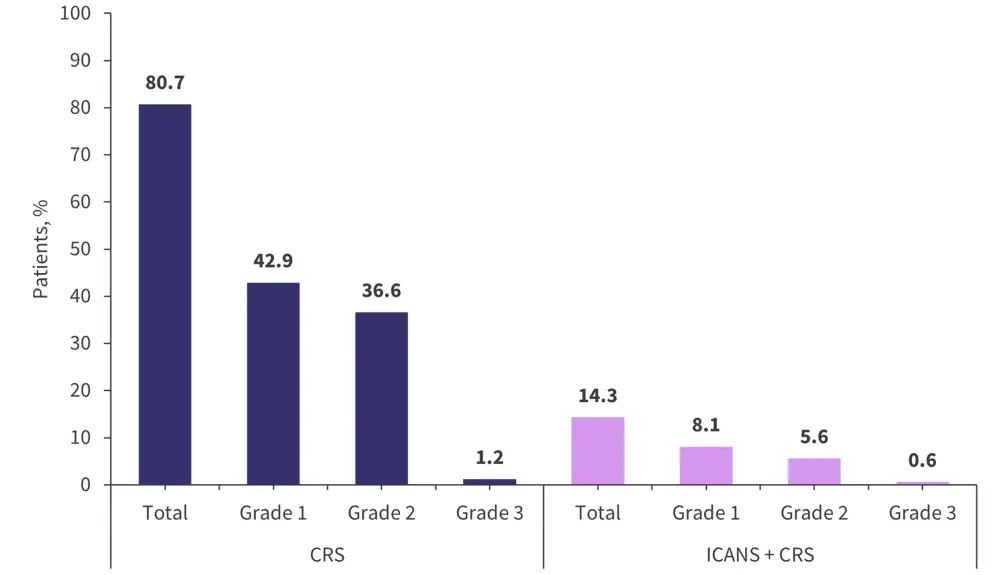
CRS, cytokine release syndrome; ICANS, immune effector cell-associated neurotoxicity syndrome.
*Data from Trudel, et al.3
In the two-dose cohorts, different CRS profiles were seen (Figure 3). In the C1 single step-up (SSU) cohort, 88.4% of patients experienced CRS compared with 79.5% in the double step-up (DSU) cohort. The DSU profile appeared to be improved, with 52.3% of patients with Grade 2–3 CRS symptoms in the DSU cohort compared with 73.3% in the SSU cohort.
Figure 3. CRS incidence in the SSU and DSU cohorts*
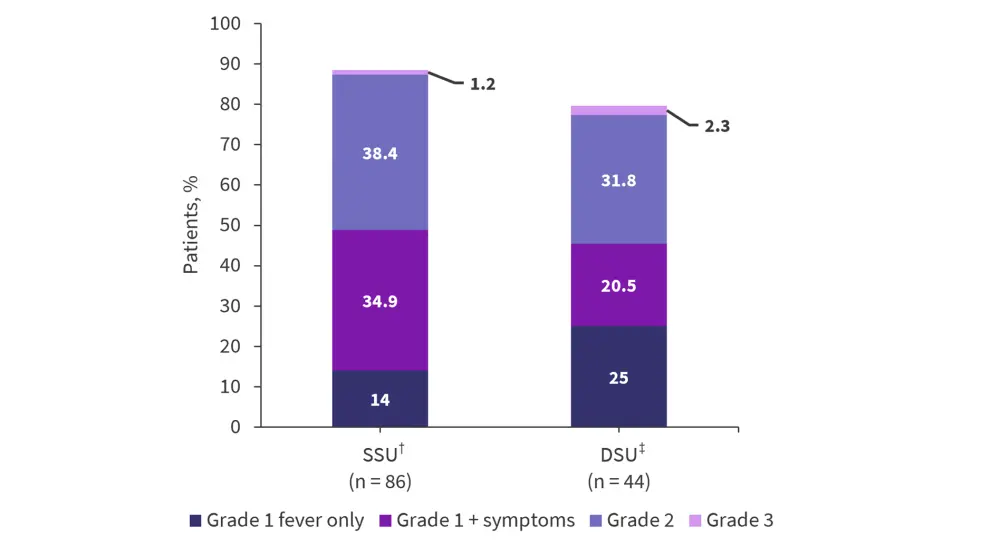
CRS, cytokine release syndrome; DSU, double step-up; SSU, single step-up.
*Adapted from Trudel, et al.3
†3.6/target dose (10.8–198 mg).
‡0.3/3.6/target dose (60–160 mg).
Efficacy
A response was observed in patients at the ≥20 mg target dose (n = 143).
Responses were more frequent at the higher dose level (132−198 mg), with an overall response rate (ORR) of 56.7%, compared with 36.1% for the lower dose cohort (20−90 mg) (Figure 4).
Figure 4. Best response to treatment*
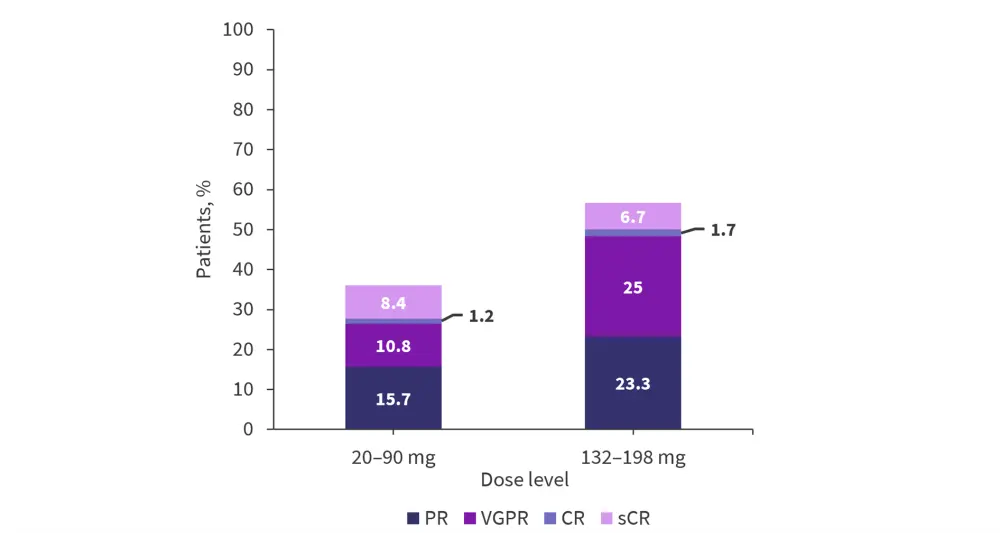
CR, complete response; PR, partial response; sCR, stringent CR; VGPR, very good partial response.
*Adapted from Trudel, et al.3
The median time to first response was 1 month (range, 0.7−5.9 months), and responses were seen to deepen over time, with a median time to best response of 2.1 months (range, 0.7−11.4 months). Only ten patients who achieved a very good partial response or better were evaluable for measurable residual disease (at 10−5 threshold), of which seven achieved measurable residual disease negativity.
Response duration
The median duration of response for the whole cohort was 11.5 months (95% confidence interval, 6.0–18.4). In the C1 SSU cohort, the median follow-up was 14.3 months (range, 2.7−31.8 months), whereas in the C1 DSU cohort, the median follow-up was 6.5 months (range, 4.8−21.4 months).
Conclusion
The safety profile of cevostamab was improved with the C1 DSU dosing schedule compared with the SSU, with a reduced CRS incidence recorded in the former. In addition, the ORR was increased at the 132−198 mg dose level compared with the 20−90 mg dose level. In this heavily pretreated cohort, which included patients with previous exposure to CD38 and BCMA-targeted agents, an ORR of 54.8% was achieved in the C1 DSU group, indicating cevostamab may be a beneficial treatment for this group and deserves further assessment in a phase II study.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?

