All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Bispecific antibodies and BiTEs currently in development for RRMM
Despite the amazing advances in treating multiple myeloma (MM), it remains incurable with a significant proportion of patients presenting with poor outcomes in the relapsed/refractory MM (RRMM) setting. More specifically, there is a considerable unmet medical need for patients who are refractory to initial salvage treatment with proteasome inhibitors (PIs), immunomodulatory drugs (IMiDs®), and/or anti-CD38 monoclonal antibodies (mAbs). From the most recent trial data, it has been estimated that patients with RRMM who are refractory to anti-CD38 mAbs and either one IMiD or PI (double-refractory) have a median overall survival (OS) of 11.2 months (95% CI, 5.4–17.1), which reduces significantly with increased refractoriness.1,2 Median OS for triple- and quad-refractory patients has been reported at 9.2 months (95% CI, 7.1–11.2), while penta-refractory patients (refractory to anti-CD38 mAbs + 2 PIs + 2 IMiDs) have a strikingly low median OS of only 5.6 months (95% CI, 3.5–7.8).1,2
Many emerging immunotherapies are currently being developed and optimized to improve survival outcomes in this patient subset and address this significant unmet medical need. These include chimeric antigen receptor T-cells (CAR-T), CAR natural killer cells (CAR-NK), antibody-drug conjugates (ADCs), and bispecific antibodies.3 The MM Hub has conducted a thorough search for all current bispecific antibodies under clinical evaluation to treat patients with RRMM. We hereby provide a summary of all available data and information on these ongoing studies. For a detailed overview of the CAR-T therapies under development for RRMM, please read here.
Bispecific antibodies
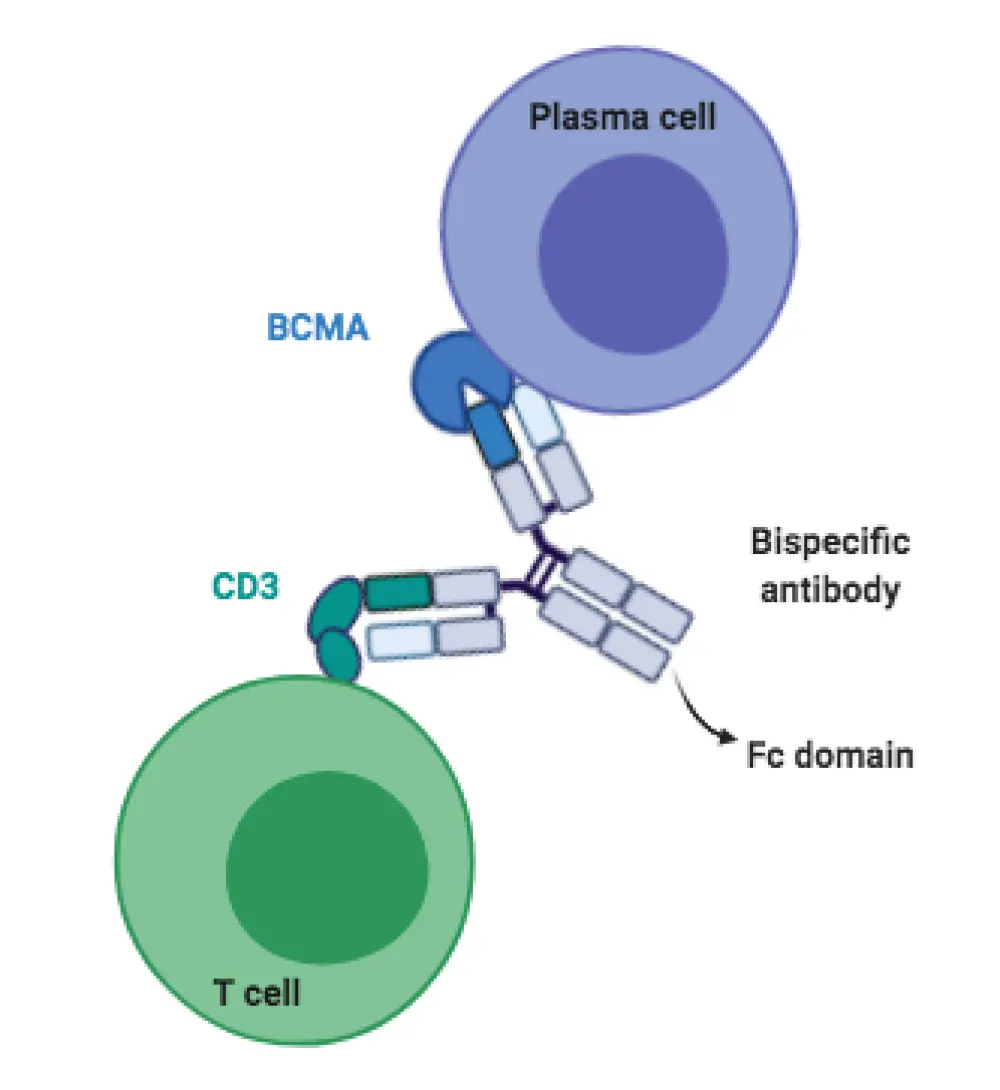
Bispecific monoclonal antibodies are engineered in such a way to direct cytotoxic T cells against tumor cells. They achieve that by having two different target-binding sites, thus simultaneously binding CD3 on T cells and a specific target tumor antigen (Figure 1).3,4 Bispecific antibodies do not require the need to extract cells from the patient, as seen with CAR therapies, and can thus be used ‘off-the-shelf’. This feature enables faster treatment delivery and bypasses laborious and expensive manufacturing steps.4 Their safety profile is similar to that of CAR therapies as they both lead to the activation of the patient’s immune cells, with the potential of cytokine release syndrome (CRS) and neurotoxicity events.3,4
Bispecific T-cell engagers (BiTEs)
Bispecific T-cell engagers (BiTEs) are a type of bispecific antibodies. They differ from the latter in their structure by lacking the Fc domain (Figure 1) and having the two different target-binding single-chain variable fragments connected by a linker.3,4 Most BiTEs have a short half-life and need continuous infusion to sustain efficacy.3 Examples of BiTEs developed for RRMM are shown in Table 1 and are analyzed further below.
Figure 1. Schematic illustration of the structure of a BCMA-targeting bispecific antibody. Bispecific T-cell engagers (BiTEs) lack the Fc domain and the target-binding fragments are connected by a linker.
Target antigens
The B-cell maturation antigen (BCMA) is considered the ‘superstar’ target antigen for MM. This is because it is widely expressed on malignant plasma cells, there is little downregulation occurring, and has no significant off-target side effects.1 BCMA is a transmembrane glycoprotein that belongs to the tumor necrosis factor receptor superfamily (TNFRSF17) and is also known as CD269. Increased levels of BCMA have been correlated to high tumor burden and poor outcomes in MM.3 The majority of bispecific and BiTEs currently under development for RRMM target BCMA and are shown in Table 1. Other tumor targets being exploited for the development of bispecific antibodies in RRMM include CD38, GPRC5d, and FcRH5 (Table 2).
Clinical trials investigating the safety and efficacy of bispecific antibodies & BiTEs
Table 1 summarizes all the BCMA-targeting bispecific antibodies and BiTEs under clinical evaluation for the treatment of RRMM. Below we discuss the compounds with preliminary results in the RRMM setting.
AMG 420 & AMG 701 BiTEs
AMG 420 was the first BiTE to be clinically evaluated in a first-in-human phase I trial (NCT02514239). In this study, 42 patients who had progressed after at least two prior lines of treatment (including both PIs and IMiDs) received continuous intravenous (i.v.) 4-week infusions of AMG 420 for a maximum of ten 6-week cycles.5 The maximum tolerated dose (MTD) was estimated at 400 μg/day, and 70% of patients achieved a response in that dose within a median time of 1 month. Of those, five were measurable residual disease (MRD)-negative stringent complete responses (sCR), one very good partial response (VGPR), and one partial response (PR).5 The responses seemed to be quite durable, with a median duration of response of 8.4 months and three of them ongoing for > 1 year.5 Serious adverse events (AEs) were observed in 48% of patients with infections being the most common. CRS was seen in 38% of patients, while no neurotoxicity was reported. Despite the promising preliminary results of this trial, the development of AMG 420 BiTE has been discontinued due to its need for continuous i.v. infusion and short half-life.1,5
Because of that, the next-generation BiTE, AMG 701, was engineered with a longer half-life and a dosing schedule of once weekly. This compound is currently being investigated in a phase I trial (NCT03287908) in patients who have progressed after ≥ 3 prior lines, including a PI, an IMiD, and anti-CD38 mAb therapy.
Table 1. BCMA-targeting bispecific antibodies in development for RRMM5,6,7,8
|
ASCO, American Society of Clinical Oncology; ASH, American Society of Hematology; BCMA, B-cell maturation agent; IMiD, immunomodulatory drugs; mAb, monoclonal antibody; MTD, maximum tolerated dose; ORR, objective response rate; PI, proteasome inhibitor; PK, pharmacokinetics; RP2D, recommended phase 2 dose; RRMM, relapsed/refractory multiple myeloma. *Double-refractory: refractory to one IMiD and one PI; Triple-refractory: refractory to one IMiD, one PI, and one anti-CD38 mAb; penta-refractory: refractory to two IMiD, two PIs, and one anti-CD38 mAb. |
||||||
|
Bispecific Ab |
MM cell target antigen |
NCT reference |
Phase |
Patient population |
Primary outcome |
Trial status |
|---|---|---|---|---|---|---|
|
AMG 420 (BiTE) |
BCMA |
I |
RRMM (36% double-refractory*)5 |
Safety/tolerability & MTD |
Completed (Data published5) |
|
|
AMG 701 (BiTE) |
BCMA |
I |
RRMM (at least triple-refractory*) |
Safety/tolerability & RP2D |
Recruiting |
|
|
CC-93269 |
BCMA |
MM-001 NCT03486067 |
I |
RRMM (66.7% triple-refractory*)6 |
Safety/tolerability (Part A) & safety/efficacy (Part B) |
Recruiting (Interim results presented at ASH 2019)6 |
|
Teclistamab (JNJ-64007957; DuoBody®) |
BCMA |
I |
RRMM (80% triple-refractory & 41% penta-refractory*)8 |
Safety/tolerability & RP2D |
Recruiting (Interim results presented at ASCO 2020)8
|
|
|
PF-06863135 |
BCMA |
I |
RRMM (at least triple-refractory*) |
Safety/tolerability & RP2D |
Recruiting |
|
|
REGN5458 |
BCMA |
I/II |
RRMM (at least triple-refractory*) |
Safety/tolerability & RP2D (phase I)
ORR (phase II) |
Recruiting (first results presented at ASH 2019)9 |
|
|
REGN5459 |
BCMA |
I/II |
RRMM (at least triple-refractory) |
Safety/tolerability, dose-limiting toxicities & RP2D |
Recruiting |
|
|
TNB-383B |
BCMA |
I |
RRMM (at least triple-refractory*) |
Safety & PK |
Recruiting
|
|
CC-93269 bispecific antibody
Another bispecific antibody with promising preliminary results is CC-93269: a BCMA-targeting bispecific that binds monovalently on T cells and bivalently on tumor cells, and is currently being investigated in the phase I MM-001 trial (NCT03486067). Interim results from this study were presented by Costa et al6 at the 2019 American Society of Hematology (ASH) meeting. Thirty patients that progressed after ≥ 3 prior lines with 66.7% of them being triple-refractory, received 2-hour weekly i.v. infusions of CC-93269 in a dosing range from 0.15–10 mg. The most common Grade ≥ 3 AEs were neutropenia, anemia, thrombocytopenia, and infections. CRS occurred in 76.7% of patients with one patient dying from CRS Grade ≥ 3.6 Preliminary efficacy data showed that the overall response rate (ORR) in all patients and doses was 43.3%, with 16.7% of patients achieving MRD-negative sCR/CR. Amongst those receiving CC-93269 at 10 mg, the ORR was 88.9%, with 44.4% achieving sCR/CR.6 Based on these promising results, enrollment continues to identify the right phase II dose. For further information on the study design and results of this study, please read here.
Teclistamab bispecific antibody
Last but not least, another noteworthy bispecific antibody with preliminary results is teclistamab (JNJ-64007957). This is a BCMA-targeting DuoBody® currently under clinical evaluation in a phase I trial (NCT03145181). The interim results were recently presented at the 2020 American Society of Clinical Oncology (ASCO) meeting.8
Of the 78 treated patients, 80% were triple-refractory and 41% penta-refractory. In the dose-escalation part of the trial (Part 1), patients received weekly i.v. injections with 1–3 step-up doses within 1 week before the full dose (doses ranged from 0.3–720 μg/kg). The most common Grade ≥ 3 AEs were neutropenia, anemia, thrombocytopenia, infections, and leukopenia. CRS occurred in 56% of patients, but none were considered Grade ≥ 3 events. Neurotoxicity was reported in six patients, with two of them being Grade ≥ 3.8 With regards to preliminary efficacy, it seems to be dose-dependent with an ORR of 30% at the 38.4–180 μg/kg dose levels and 67% at the 270 μg/kg dose. Efficacy data for the 720 μg/kg dose have not yet been presented. The responses seem to deepen over time, with 76% of responders still having ongoing responses.8 Further data from the dose-escalation and expansion parts of the study (Part 2) are soon awaited.
Non-BCMA bispecific antibodies
Table 2 below provides a summary of all the ongoing trials that are evaluating a non-BCMA targeting bispecific antibody. No data is available yet for any of these products; thus, their feasibility and safety in patients with RRMM still needs to be confirmed.
Table 2. Non-BCMA targeting bispecific antibodies in development for RRMM7
|
FIH, first-in-human; mAb, monoclonal antibody; MTD, maximum tolerated dose; ORR, objective response rate; RP2D, recommended phase 2 dose; RRMM, relapsed/refractory multiple myeloma. *Double-refractory: refractory to one IMiD and one PI; Triple-refractory: refractory to one IMiD, one PI, and one anti-CD38 mAb. |
||||||
|
Bispecific Ab name |
MM cell target antigen |
Study name/NCT reference |
Phase |
Patient population |
Primary outcome |
Trial status |
|---|---|---|---|---|---|---|
|
Talquetamab (JNJ- 64407564) |
GPRC5d |
I |
RRMM |
Safety/tolerability & RP2D |
Recruiting |
|
|
GBR 1342 |
CD38 |
I/II |
RRMM (at least triple-refractory*) |
Safety/tolerability & ORR |
Recruiting |
|
|
AMG 424 |
CD38 |
I (FIH) |
RRMM (at least double-refractory*) |
Safety/tolerability & MTD |
Terminated by sponsor |
|
|
BFCR4350A |
FcRH5 |
I |
RRMM |
Safety |
Recruiting |
|
Conclusion
To date, there is a scarce clinical experience with bispecific antibody therapy for RRMM. Nevertheless, preliminary data from various BCMA-targeting bispecific antibodies and BiTEs indicate that they present a feasible therapeutic option. Although their complete efficacy and safety profile is yet to be established, they show great potential for the treatment of double- to penta-refractory RRMM patients and provide hope for this patient subset. As there are no guidelines regarding the best indications for using bispecific antibodies, future studies are needed to validate their potential use in other MM settings, like high- and ultra-high-risk MM.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?

