All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Attenuated responses to COVID-19 vaccination in patients with hematologic malignancies
Patients with hematologic malignancies have an increased risk of infections, due to disease-related immune dysregulation combined with the immunosuppressive effect of some therapies. It is therefore considered particularly important to vaccinate this vulnerable patient population against COVID-19. The National Comprehensive Cancer Network (NCCN) have recently published guidelines for COVID-19 vaccination in patients with hematologic malignancies, summarized previously on the Multiple Myeloma Hub.
Response to vaccination is often attenuated in immunocompromised patients but the efficacy of COVID-19 vaccines in patients with hematologic malignancies is uncertain. Here, we summarize four studies reporting on the response to vaccination against SARS-CoV-2 in subgroups of patients with specific hematologic malignancies, namely multiple myeloma (MM), myeloproliferative malignancies (MPM), and chronic lymphocytic leukemia (CLL).1–4
Response to COVID-19 vaccination in multiple myeloma
In a retrospective UK study by Sarah Bird and colleagues,1 serological responses were evaluated in 93 patients with MM following the first dose of either AZD1222 or BNT162b2 SARS-CoV-2 vaccine.
In total, 56% of patients were positive for SARS-CoV-2 immunoglobulin (Ig)G antibodies ≥21 days after vaccination, lower than observed in local testing of hospital staff using the same antibody tests (99% positivity rate). There was no difference in number of seropositive patients according to vaccine type (AZD1222 or BNT162b2), supporting current UK recommendations to vaccinate with whichever vaccine is available.1
Factors associated with lower seropositive rates (Table 1) were:
- Active disease (stable or progressive)
- Immunoparesis at the time of vaccination
- More previous lines of therapy
- Being on therapy at the time of vaccination
Table 1. Factors associated with anti-SARS-CoV-2 IgG antibody response in patients with MM*
|
CR, complete response; IMWG, International Myeloma Working Group; PD, progressive disease; PR, partial response; SD, stable disease; VGPR, very good partial response. |
|||
|
|
Positive antibody result, n (%) |
Negative antibody result, n (%) |
p value |
|---|---|---|---|
|
Disease status (IMWG criteria) |
|
|
0.0046 |
|
CR or VGPR (n = 48) |
30 (63) |
18 (38) |
|
|
PR (n = 16) |
12 (75) |
4 (25) |
|
|
SD or PD (n = 27) |
8 (30) |
19 (70) |
|
|
Unable to assess (n = 2) |
2 (100) |
0 (0) |
|
|
Immunoparesis |
|
|
0.039 |
|
Yes (n = 43) |
19 (44) |
24 (56) |
|
|
No (n = 50) |
33 (66) |
17 (34) |
|
|
Previous lines of therapy, n (range) |
1 (0–3) |
1 (0–8) |
0.0059 |
|
Therapy status at time of vaccination |
|
|
0.037 |
|
On therapy (n = 66) |
32 (48) |
34 (52) |
|
|
Not on therapy (n = 27) |
20 (74) |
7 (26) |
|
There was no difference in seropositivity rates according to type of treatment and, importantly, seropositivity rates for patients receiving autologous stem cell transplantation (ASCT) were high (75% of patients who were vaccinated within 12 months of ASCT had positive antibodies; all had at least a partial response).1
It is worth noting that these results are based on anti-SARS-CoV-2 IgG antibody responses, which are not equivalent to neutralizing antibodies, and the degree of association between IgG response and clinical protection is undetermined. When anti-SARS-CoV-2 total antibody levels (IgG, IgA, and IgM) were assessed, the positive antibody response rose from 56% to 70%.1
In a study by Evangelos Terpos and colleagues,2 neutralizing antibody responses to SARS-CoV-2 were similarly found to be attenuated in elderly patients with MM after an initial dose of the BNT162b2 vaccine. Neutralizing antibody titers on Day 22 after the first vaccine dose were compared between 48 patients with MM (median age, 83 years; range, 59–92) and 104 volunteers of similar age and gender. The following observations were made:
- Patients with MM had lower neutralizing antibody titers than controls (median, 20.6% vs 32.5%; p < 0.01)
- Only 25.0% of patients with MM developed titers ≥30% (the cut-off defining antibody positivity), compared with 54.8% of controls
- Only 8.3% of patients with MM developed titers ≥50% (the level corresponding to clinically relevant viral inhibition), compared with 20.2% of controls
The four patients with MM who developed neutralizing antibody titers ≥50% were in remission, off therapy, with normal uninvolved immunoglobulins.2 These results are consistent with the findings from the previous study that antibody response is associated with disease activity, treatment status, and immune status.
In a study of 42 patients on active treatment for myeloma,3 antibody responses were found to be strengthened following a second dose of the BNT162b2 vaccine. The SARS-CoV-2 IgG antibody positivity rate was just 21.4% at 21 days after the first dose but increased to 78.6% by 14 days after the second dose (5 weeks after the initial vaccination). Although increased, this positivity rate was significantly inferior to an elderly control population (100%, p = 0.003).
Taken together, these data suggest that most patients with MM should have some protection against SARS-CoV-2 after the first vaccination, although antibody responses are likely to be attenuated. Protection is expected to increase after a second vaccination, and timely administration of the second dose will therefore be critical. It will also be imperative to closely monitor this high-risk patient group, as non-responders may be more vulnerable to severe COVID-19 and may need to take extra precautions to minimize risk of infection.
Response to COVID-19 vaccination in myeloproliferative malignancies3
In a subgroup of 50 patients with MPM (20 patients with chronic myeloid leukemia and 30 patients with myeloproliferative neoplasms), Pimpinelli and colleagues observed a greater SARS-CoV-2 IgG antibody response, by both geometric mean concentration (GMC) and seropositivity rate, after two doses of the BNT162b2 vaccine than that seen for patients with MM. However, responses were still attenuated compared with those seen in an elderly control population (Figure 1; p = 0.049 for GMC and p = 0.038 for seropositivity rate vs control). Interestingly, after a single vaccine dose, the response for patients with MPM was not statistically different to the control population (GMC, 16.2 AU/mL vs 17.1 AU/mL; seropositivity rate, 52% vs 52.8%).
Figure 1. Anti-SARS-CoV-2 IgG geometric mean concentrations and seropositivity rates before and after first and second vaccine dose in patients with MM and MPM in comparison with elderly controls*
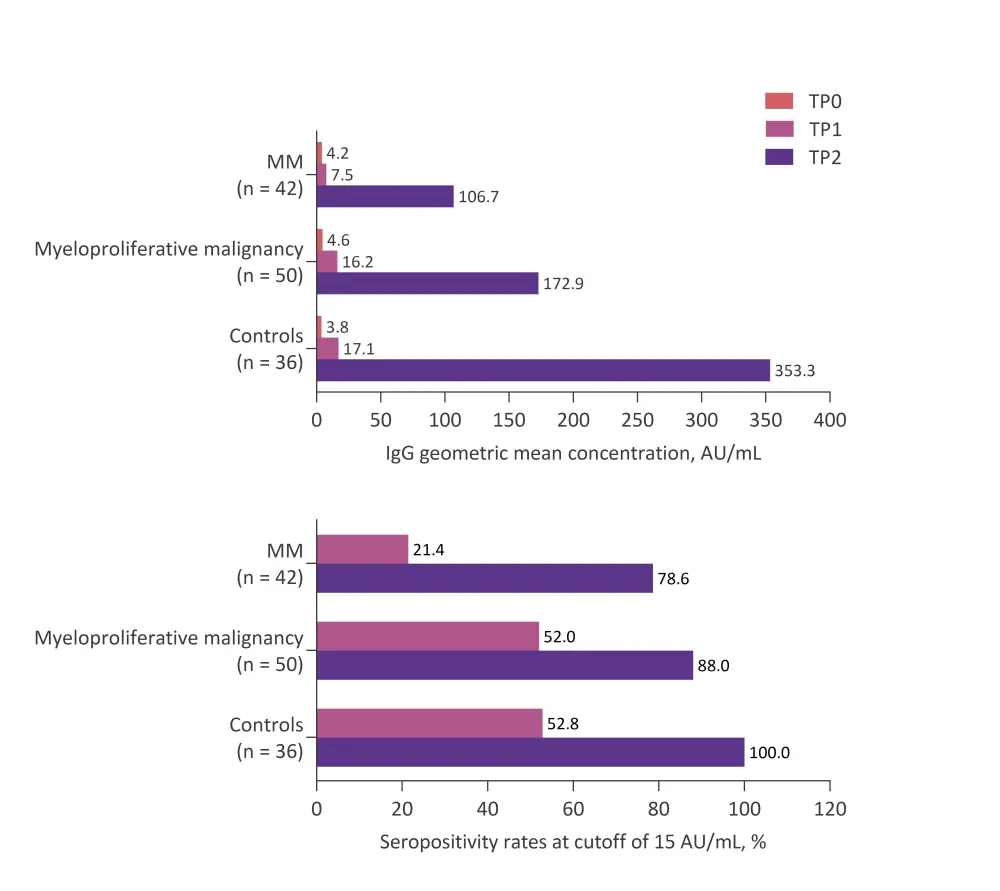
MM, multiple myeloma; TP0, day of first vaccine dose; TP1, day of second vaccine dose (third week after first dose); TP2, fifth week after first dose.
*Adapted from Pimpinelli et al.3
Only six patients in the MPM cohort failed to achieve seropositivity above the 15 AU/mL cut-off, including four of eight patients with myelofibrosis. Despite small numbers, the authors consider that myelofibrosis may therefore be associated with a lower likelihood of response to vaccination. The other non-responders were one patient with essential thrombocythemia, and one with polycythemia vera.
Response to COVID-19 vaccination in CLL4
Yair Herishanu and colleagues assessed antibody responses to the BNT162b2 SARS-CoV-2 vaccine in a total of 167 patients with CLL. In this study, serologic responses were assessed after two doses of vaccine, administered 21 days apart.
Like those observed for patients with MM, antibody responses to SARS-CoV-2 vaccination were low overall for patients with CLL (39.5%), and significantly lower compared with age- and gender-matched controls (52% [n = 52] vs 100% [n = 52]; p < 0.001). Responses were similarly influenced by disease stage, treatment status, and immunoparesis, as well as by age, gender, IGHV mutational status, and β2-microglobulin levels in univariate analysis (Table 3). Factors which remained significant by multivariate analysis were age ≤65 years (p = 0.025), female gender (p = 0.006), lack of active therapy (p < 0.001), serum IgG ≥550 mg/dL (p = 0.037), and serum IgM ≥40 mg/dL (p = 0.017).
Table 2. Factors associated with anti-SARS-CoV-2 IgG antibody response in patients with CLL in univariate analysis*
|
Ig, immunoglobulin; IGHV, immunoglobulin heavy chain variable region. |
|||
|
|
Positive antibody result, n (%) |
Negative antibody result, n (%) |
p value |
|---|---|---|---|
|
Age at time of vaccination |
|
|
0.031 |
|
≤65 years (n = 50) |
26 (52.0) |
24 (48.0) |
|
|
>65 years (n = 117) |
40 (34.2) |
77 (65.8) |
|
|
Gender |
|
|
0.005 |
|
Male (n = 55) |
30 (54.5) |
25 (45.5) |
|
|
Female (n = 112) |
36 (32.1) |
76 (67.9) |
|
|
Treatment status |
|
|
<0.001 |
|
Treatment naïve (n = 58) |
32 (55.2) |
26 (44.8) |
|
|
On therapy (n = 75) |
12 (16.0) |
63 (84.0) |
|
|
Off therapy in remission (CR/PR, n = 24) |
19 (79.2) |
5 (20.8) |
|
|
Off therapy in relapse (n = 10) |
3 (30.0) |
7 (70.0) |
|
|
Binet Stage |
|
|
0.001 |
|
A (n = 43) |
29 (67.4) |
14 (32.6) |
|
|
B or C (n = 24) |
6 (24.0) |
18 (75.0) |
|
|
IGHV |
|
|
0.005 |
|
Mutated (n = 61) |
29 (47.5) |
32 (52.5) |
|
|
Unmutated (n = 61) |
14 (23.0) |
47 (77.0) |
|
|
β2-microglobulin |
|
|
0.004 |
|
≤3.5 mg/L (n = 90) |
43 (47.8) |
47 (52.2) |
|
|
>3.5 mg/L (n = 25) |
4 (16.0) |
21 (84.0) |
|
|
Current treatment status |
|
|
<0.001 |
|
Untreated (n = 92) |
54 (58.7) |
38 (41.3) |
|
|
Treated (n = 75) |
12 (16.0) |
63 (84.0) |
|
|
Last anti-CD20 treatment |
|
|
<0.001 |
|
≥12 months before vaccination (n = 55) |
25 (45.5) |
30 (54.6) |
|
|
<12 months before vaccination (n = 22) |
0 (0.0) |
22 (100.0) |
|
|
Serum IgG |
|
|
<0.001 |
|
≥550 mg/dL (n = 108) |
53 (49.1) |
55 (50.1) |
|
|
<550 mg/dL (n = 46) |
7 (14.6) |
39 (85.4) |
|
|
Serum IgM |
|
|
<0.001 |
|
≥40 mg/dL (n = 66) |
39 (59.1) |
27 (40.9) |
|
|
<40 mg/dL (n = 87) |
20 (23.0) |
67 (77.0) |
|
|
Serum IgA |
|
|
0.012 |
|
≥80 mg/dL (n = 89) |
42 (34.5) |
47 (54.5) |
|
|
<80 mg/dL (n = 63) |
17 (24.5) |
46 (38.5) |
|
Considering treatment status, responses were highest for patients who completed treatment and remained in remission (79.2%), and lowest for patients receiving therapy at the time of vaccination (16.0%). Responses were particularly low for patients receiving either Bruton’s tyrosine kinase inhibitors (16.0%) or venetoclax +/- anti-CD20 antibody therapy (13.6%), and patients treated with an anti-CD20 therapy (rituximab or obinutuzumab) within 12 months of vaccination (n = 22) failed to respond. Given these results, the authors propose that it may be appropriate to consider delaying the start of treatment to allow completion of the vaccination program, if deemed safe for the patient.
Summary
Close follow-up of immunocompromised patients, such as those with hematologic malignancies, will be critical after COVID-19 vaccination since attenuated vaccine responses may leave them vulnerable to COVID-19 infection. Booster vaccine doses may be necessary to ensure adequate protection, although further research is needed to establish the optimal timing of these additional doses. One limitation to the studies evaluating anti-SARS-CoV-2 IgG antibodies is that they are not equivalent to neutralizing antibodies, and the degree of association between IgG response and clinical protection is uncertain. Larger studies with longer follow-up are urgently needed to better understand the protective effects of vaccination in patients with MM, CLL, and other hematologic malignancies.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?

