All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Allogeneic CAR T-cell therapy for relapsed or refractory multiple myeloma: Results from the UNIVERSAL trial
Therapy with B-cell maturation antigen (BCMA)-directed autologous chimeric antigen receptor (CAR) T-cells has demonstrated encouraging efficacy in patients with relapsed/refractory (R/R) multiple myeloma (MM). However, a number of factors limit the availability of autologous therapies, such as the quantity and quality of cells collected or the extended vein-to-vein time. Allogeneic (allo) cell therapies have been proposed as an alternative, with the aim to overcome manufacturing- and patient-associated challenges.
ALLO-715 is an anti-BCMA allo-CAR T-cell product that, importantly, lacks CD52. This attribute allows for lymphodepletion with the CD52-targeting monoclonal antibody ALLO-647 with no impact on the therapeutic T-cells. Furthermore, ALLO-715 lacks the T-cell receptor alpha constant gene, making patients less susceptible to developing graft-versus-host disease.
During the 62nd American Society of Hematology (ASH) Annual Meeting and Exposition, Sham Mailankody presented the data from the first-in-human UNIVERSAL study (NCT04093596) of ALLO-715 in combination with ALLO-647 lymphodepletion in patients with R/R MM.1 The Multiple Myeloma Hub is pleased to present a summary.
Study design
Patients with MM who were R/R to their last prior therapy were eligible for enrollment if they met the following criteria:
- Three or more prior therapies with an immunomodulatory drug, proteasome inhibitor, and an anti-CD38 therapy
- Eastern Cooperative Oncology Group (ECOG) performance score of 0 or 1
- Absence of donor-specific antibodies
- Bridging therapy was not allowed.
The UNIVERSAL study schema is outlined in Figure 1. The primary endpoints of the study were safety and tolerability, and secondary endpoints included:
- Recommended phase II dose of ALLO-715 and ALLO-647 lymphodepletion regimen
- Overall response rate, duration of response, progression-free survival, and measurable residual disease
- Cellular kinetics of ALLO-715
- Pharmacokinetic profile of ALLO-647.
Figure 1. Study schema for the UNIVERSAL study1
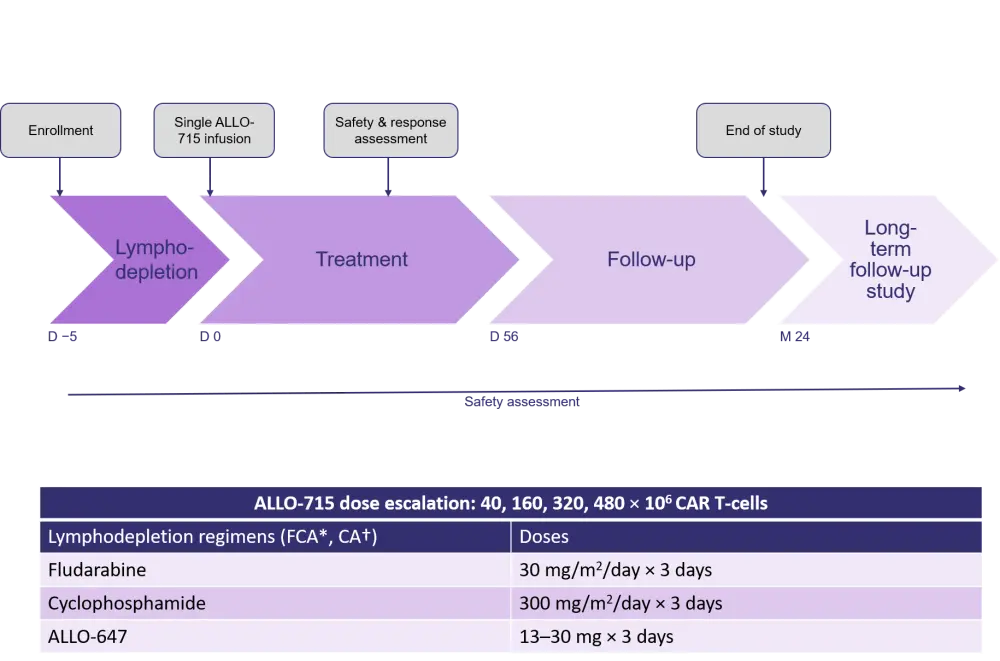
CAR, chimeric antigen receptor; D, day; M, month.
*FCA conditioning with fludarabine, cyclophosphamide and ALLO-647; †CA conditioning with cyclophosphamide and ALLO-647.
Results
- Median follow-up: 3.2 months.
- At the data cut-off, 31 and 26 patients comprised the safety and efficacy populations, respectively.
Patient characteristics
The study population (N = 31) was largely comprised of patients with heavily pretreated, advanced, refractory stage MM (Table 1).
Table 1. Patient characteristics1
|
Auto-SCT, autologous stem cell transplant; ECOG, Eastern Cooperative Oncology Group; ISS, International Scoring System. *del17p, t(4;14), t(14;16); †received therapy with ≥2 immunomodulatory drugs, ≥2 proteasome inhibitors, and a CD38 monoclonal antibody. |
|
|
Characteristics, % unless stated otherwise |
Safety population (N = 31) |
|---|---|
|
Median age, years (range) |
65 (46–76) |
|
Male |
61 |
|
ECOG performance score |
|
|
0 |
48 |
|
1 |
52 |
|
ISS stage ≥2 |
74 |
|
High-risk cytogenetics* |
48 |
|
Median prior lines of therapy, n |
5 |
|
Prior auto-SCT |
94 |
|
Penta-exposed† |
94 |
Efficacy
From the early efficacy data, no objective responses were observed at dose level 1, but response rates improved in patients receiving dose level 3 and FCA conditioning (Table 2). Of the six patients who achieved a very good partial response or better, five were analyzed for measurable residual disease, all of whom were determined negative by multicolor flow cytometry.
Table 2. Response rates to variable doses of ALLO-7151
|
CA, conditioning with cyclophosphamide and ALLO-647; DL, dose level; FCA, conditioning with fludarabine, cyclophosphamide, and ALLO-647; M, million; ORR, overall response rate; VGPR, very good partial response. |
||||||
|
ALLO-715 cell dose and LD regimen |
FCA |
CA |
||||
|---|---|---|---|---|---|---|
|
DL2 (160M) |
DL3 (320M) |
DL4 (480M) |
DL3 (320M) |
|||
|
Low |
Low |
High |
All |
Low |
Low |
|
|
ORR, % |
50 |
50 |
75 |
60 |
33 |
67 |
|
≥VGPR, % |
25 |
50 |
25 |
40 |
— |
33 |
Safety
The combination of ALLO-715 and ALLO-647 exhibited a promising safety profile, with no graft-versus-host disease or immune effector cell‐associated neurotoxicity syndrome reported. Furthermore, cytokine release syndrome was well managed with tocilizumab and steroids, and infusion-related reactions were all low grade (1 or 2). Grade ≥3 adverse events were observed in 19% of patients and there was one Grade 5 event resulting from progressive myeloma in the conditioning with cyclophosphamide and ALLO-647 cohort.
CAR T-cell expansion
ALLO-715 CAR T-cell expansion was observed by Day 7, increased respective of dose, and persisted for 4 months in patients who received dose level 3.
Conclusion
The UNIVERSAL trial represents the first clinical trial to evaluate an allo-BCMA-targeted CAR T-cell product. Preliminary data suggest that ALLO-715 demonstrates a promising efficacy and safety profile in patients with heavily pretreated R/R MM. Enrollment for the planned evaluation of increasing cell doses and optimization of lymphodepletion regimens is ongoing.
Allo-CAR T-cell therapy is not limited to the MM setting. The CD19-directed therapy, ALLO-501, appears well tolerated and demonstrated encouraging short-term preliminary efficacy in patients with R/R diffuse large B-cell lymphoma or follicular lymphoma. You can read more here.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?

