All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The mm Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the mm Hub cannot guarantee the accuracy of translated content. The mm and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The Multiple Myeloma Hub is an independent medical education platform, sponsored by Bristol Myers Squibb, GSK, Legend Biotech, Pfizer, and Roche. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View multiple myeloma content recommended for you
Advances of cell therapies in China: Reducing manufacturing time with FasT dual CAR T-cells
The Multiple Myeloma Hub previously summarised activities on chimeric antigen receptor (CAR) T cells in China. Jianxiang Wang presented this topic at the 2nd European CAR T-cell Meeting, which has been summarised here. The article provided a summary of some of the investigational new drugs (IND) developed in China at the time.
At the 3rd European CAR T-cell Meeting, held virtually in February 2021, Jianxiang Wang presented information on the progression of CAR-T trials in China, specifically on reducing manufacturing times (FasTCAR) and incorporating a second target (dual CAR). He reported results from the trials evaluating the efficacy of GC022F and GC012F in relapsed/refractory (R/R) B-cell acute lymphoblastic leukemia (B-ALL) and multiple myeloma (MM), respectively.1
Overview of clinical research with CAR T-cells in China
- China is leading in CAR T-cell research with 265 ongoing clinical trials, mostly investigator-initiated studies, followed by 217 trials in the US
- Thirteen industries are involved in a total of 21 IND trials, including five phase II trials
- Of these five trials, three are in non-Hodgkin lymphoma (NHL), one in MM, and one in ALL
- The indications for these trials included B-cell NHL (57%), myeloma (19%), leukemia (19%), and solid tumor (5%). Almost 74% of the trials were performed with cell therapy targeting CD19
Key advantages of FasT CAR T-cells2
- FasTCARTM is a registered manufacturing platform that enables a concurrent activation and transduction of T cells with an optimized infection process that also avoids the need for ex vivo cell expansion. Altogether, it significantly shortens this part of the manufacturing process from 9−14 days to 22−36 hours
- Preclinical studies with the FasTCAR technology have demonstrated that these CAR T-cells are superior in expansion and memory cell formation and reduce T-cell exhaustion, which ultimately could lead to more effective and durable treatments than the conventional CAR T-cell therapy
- Reducing the timelines and processes could also help make these cutting-edge cell therapies a more affordable treatment option
- FasTCAR products are being evaluated as therapeutic candidates for patients with MM, B-ALL, B-NHL, and solid tumors such as ovarian or breast cancer
GC022F – CD19/CD22 FasT dual CAR-T cell in R/R B-ALL
Study design
- This is a single-center phase I clinical trial (NCT04129099) evaluating the safety and tolerance of GC022F FasT CAR T-cell product targeting CD19 and CD22 in R/R B-ALL
- Enrolled patients (n = 11) were given a single dose of GC022F as follows:
- Low dose with median of 6.25 × 104/kg (6.03−6.46 × 104/kg) (n = 2)
- Medium dose with median of 1.49 × 105/kg (1.00−1.53 × 105/kg) (n = 7)
- High dose with median of 2.28 × 105/kg (2.28−2.28 × 105/kg) (n = 2)
- Primary endpoint was the incidence and severity of adverse events, cytokine release syndrome (CRS), and neurotoxicity
Results
The data cutoff date was November 4, 2020. The median follow-up was 126 days (range, 14−279 days). There was a transduction efficiency of 29.8% (17.0−60.1%) with the optimized manufacturing process and a 100% manufacture success rate.
Patient baseline characteristics
The median bone marrow (BM) blasts was 42% (range, 0−73%). See further details on baseline characteristics in Table 1.
Table 1. Baseline characteristics*
|
allo-HSCT, allogeneic hematopoietic stem cell transplant; BM, bone marrow; CAR-T, chimeric antigen receptor T-cell therapy. |
|
|
Characteristic |
n = 11 |
|---|---|
|
Gender (M/F) |
6/5 |
|
Median age, years (range) |
11 (3−48) |
|
BM Blasts at enrollment |
|
|
≥ 60%, n (%) |
2 (18.2) |
|
30−59%, n (%) |
4 (36.4) |
|
5−29%, n (%) |
1 (9.1) |
|
< 5%, n (%) |
4 (36.4) |
|
Relapsed after allo-HSCT, n |
1 |
|
Previous CD19 CAR-T, n |
3 |
|
Both CD19 CAR-T and allo-HSCT, n |
1 |
Efficacy
- In four patients with BM blasts < 5%, three patients achieved minimal residual disease (MRD) negativity on Day 28, except one, who showed a persistent MRD positivity on day 14
- Of the seven patients with BM blasts > 5%, six achieved MRD negativity
- Six MRD-negative patients followed allogeneic hematopoietic stem cell transplant (allo-HSCT), and all maintained a MRD-negative complete response (CR) except one, who died of graft-versus-host disease (GvHD), and infection
- Of the other three MRD-negative CR patients without allo-HSCT, one is still MRD negative at 4 months, another relapsed at 2.6 months, and the last one had MRD-positive recurrence at 4 months
- All relapsed patients were CD19 and CD22 positive
Safety
- The most common adverse events included leukopenia, lymphopenia, thrombocytopenia, anemia, neutropenia, and fever. See Table 2 for further details.
- The median time of onset for CRS was 6 days (range, 3−19 days), and the median duration was 8 days (range, 3−11). Only one patient (9%) developed Grade ≥ 3 CRS
- Grade 1 immune effector cell-associated neurotoxicity (ICANS) was observed in only two (18%) patients
Table 2. Safety*
|
ALT, alanine aminotransferase; AST, aspartate aminotransferase. |
||
|
Adverse event (≥ 25% All Grade), n (%) |
All patients (n = 11) |
|
|---|---|---|
|
All Grades |
Grade ≥ 3 |
|
|
Leukopenia |
11 (100) |
11 (100) |
|
Lymphopenia |
10 (91) |
10 (91) |
|
Thrombocytopenia |
9 (82) |
3 (27) |
|
Anemia |
9 (82) |
4 (36) |
|
Neutropenia |
8 (73) |
8 (73) |
|
Fever |
7 (64) |
1 (9) |
|
Hypocalcemia |
4 (36) |
0 (0) |
|
Hypokalemia |
4 (36) |
0 (0) |
|
Elevated ALT/AST |
3 (27)/3 (27) |
0 (0) |
GC012F – BCMA/CD19 FasT dual CAR T-cell for R/R MM
Study design
- Multicentre prospective, open-label study (NCT04236011) to determine the safety and efficacy of GC012F CAR T-cells in patients diagnosed with R/R MM and with B-cell maturation antigen (BCMA) expression
- Patients received the following dose levels of GC012F in a single infusion. See Figure 1 for details
-
- Dose level 1: 1 × 105 cells/kg
- Dose level 2: 2 × 105 cells/kg
- Dose level 3: 3 × 105 cells/kg
- The primary endpoint is the incidence and severity of adverse events following GC012F infusion
Figure 1. Dose levels of GC012F*
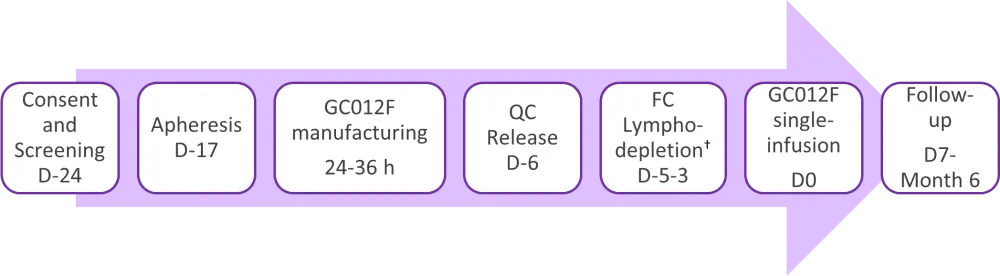
C, cyclophosphamide; D, day; F, fludarabine; h, hours; QC, quality control.
*Adapted from Wang J. 20211
†Lymphodepletion regime was 30 mg/m2/day fludarabine and 300 mg/m2/day cyclophosphamide for 3 days.
Results
Patient baseline characteristics
- Fifteen (15/16) patients had a high-risk profile, and five (5/16) had ≥1 extramedullary plasmacytoma. See Table 3.
- Enrolled patients were heavily pretreated, with a median of 5 (2−9) prior regimens, and the vast majority (94%) were exposed to proteasome inhibitors (PIs), immunomodulatory drugs (IMiDs), and anti-CD38 therapy.
Table 3. Patient baseline characteristics*
|
Auto-SCT, autologous hematopoietic stem-cell; IMiD, immunomodulatory drugs; PI, proteasome inhibitor. |
|
|
Characteristic |
n = 16 |
|---|---|
|
Median age, years (range) |
56 (27−71) |
|
Male, n (%) |
10 (63) |
|
Type, n (%) |
|
|
IgG |
7 (44) |
|
IgA |
4 (25) |
|
IgD |
3 (19) |
|
Light chain |
2 (13) |
|
Median years since diagnosis (range) |
3 (1−10) |
|
High-risk profile†, n (%) |
15 (94) |
|
Double-hit‡, n (%) |
3 (19) |
|
Extramedullary plasmacytomas ≥ 1, n (%) |
5 (31) |
|
Median prior regimens of therapy, n (range) |
5 (2−9) |
|
Median prior lines of therapy, n (range) |
5 (2−7) |
|
Prior auto-SCT, n (%) |
4 (25) |
|
Triple exposed§,ǁ, n (%) |
15 (94) |
|
PI refractory |
15 (94) |
|
IMiD refractory |
14 (88) |
|
Anti-CD38 refractory |
4 (25) |
|
Penta exposedǁ, n (%) |
10 (63) |
|
Primary refractory, n (%) |
3 (19) |
|
Refractory to last therapy, n (%) |
12 (75) |
Efficacy
- The median follow-up was 7.3 months (range, 1−10 months). Only one of the 16 patients enrolled did not achieve a response to treatment
- ORR was achieved in 15 out of 16 patients (94%) with a very good partial response (VGPR) or better
- CR or stringent CR (sCR) was achieved in nine out of 16 patients (56%)
- Six patients out of six (100%) in Dose Level 3 achieved sCR
- Median duration of response (DoR) has not been achieved yet
- All evaluable patients were MRD negative at a minimum of 10-4 sensitivity by next-generation flow at 3 months (n = 11) and 6 months (n = 10)
Safety
- The most common adverse events (AEs) included lymphopenia, neutropenia, leukopenia, and thrombocytopenia. See Table 4
- Two patients (12%) experienced Grade 3 CRS. The remaining 14 patients (88%) showed Grade 1-2 CRS
- None of the patients developed ICANS
Table 4. Safety*
|
AST, aspartate aminotransferase; LDH, lactate dehydrogenase; TEAE, treatment-emergent adverse event. |
||
|
Adverse event |
All Grades, n (%) |
Grade ≥3, n (%) |
|---|---|---|
|
Hematologic TEAE (≥25% All grades) |
||
|
Lymphopenia |
13 (81) |
13 (81) |
|
Neutropenia |
13 (81) |
13 (81) |
|
Leukopenia |
11 (69) |
11 (69) |
|
Thrombocytopenia |
11 (69) |
11 (69) |
|
Anemia |
8 (50) |
7 (44) |
|
Hypoalbuminemia |
8 (50) |
0 (0) |
|
Nonhematologic TEAE (≥25% All grades) |
||
|
LDH increase |
11 (69) |
0 (0) |
|
AST increase |
7 (44) |
5 (31) |
|
Diarrhea |
4 (25) |
0 (0) |
|
Lower respiratory tract infection |
3 (19) |
3 (19) |
Conclusion
The preclinical findings suggest that the FasTCAR platform can successfully manufacture the necessary product in 1 day with younger, less exhausted T cells and improved efficacy than conventional dual CAR-T cells.
Early clinical data for the CD19/CD22 FasT dual CAR T-cell GC022F, shows a favorable safety profile and good efficacy in treating R/R B-ALL patients.
Preliminary clinical data with the BCMA/CD19 FasT dual CAR T-cell GC012F, also showed a favorable safety profile, and promising efficacy in treating relapsed MM refractory compared with the currently available therapies.
Further studies with a larger sample size and longer-term follow-up are needed with both FasT dual CAR T-cell products.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content
Your opinion matters
On average, how many patients with MGUS/smoldering MM do you see in a month?

