All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The Multiple Myeloma Hub uses cookies on this website. They help us give you the best online experience. By continuing to use our website without changing your cookie settings, you agree to our use of cookies in accordance with our updated Cookie Policy
Introducing

Now you can personalise
your Multiple Myeloma Hub experience!
Bookmark content to read later
Select your specific areas of interest
View content recommended for you
Find out moreThe Multiple Myeloma Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the Multiple Myeloma Hub cannot guarantee the accuracy of translated content. The Multiple Myeloma Hub and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
Initial results in RRMM with modakafusp alfa: a first-in-class CD38-targeted immunocytokine
Bookmark this article
Modakafusp alfa is a first-in-class antibody–cytokine fusion protein (immunocytokine) consisting of two attenuated IFNα2b molecules genetically fused to the Fc portion of a humanized, anti-CD38, IgG4 monoclonal antibody (mAb).1 The INF-α fusion protein exhibits a 10,000-fold greater specificity for CD38+ cells versus CD38− when compared with native INF-α.2
Mechanism of action
Modakafusp alfa, previously known as TAK-573, selectively binds to a unique epitope on CD38, different to the binding epitope of the anti-CD38 mAbs approved for the treatment of multiple myeloma (MM), i.e., daratumumab and isatuximab.1,2 Through the INF-α receptor, modakafusp alfa directs anti-proliferative/apoptotic signals to tumor cells, inducing tumor cell death.2 Besides the anti-myeloma activity, modakafusp alfa also activates innate and adaptive immune cells.2 It is hypothesized that the activated immune cells and dead myeloma cells can produce a synergistic effect in destroying more malignant plasma cells in the bone marrow (Figure 1).2 Furthermore, the specificity of targeting interferon directly to the myeloma cells reduces the potential risk for off-target binding and toxicity in patients with relapsed/refractory multiple myeloma (RRMM).1
Figure 1. Proposed mechanism of action*
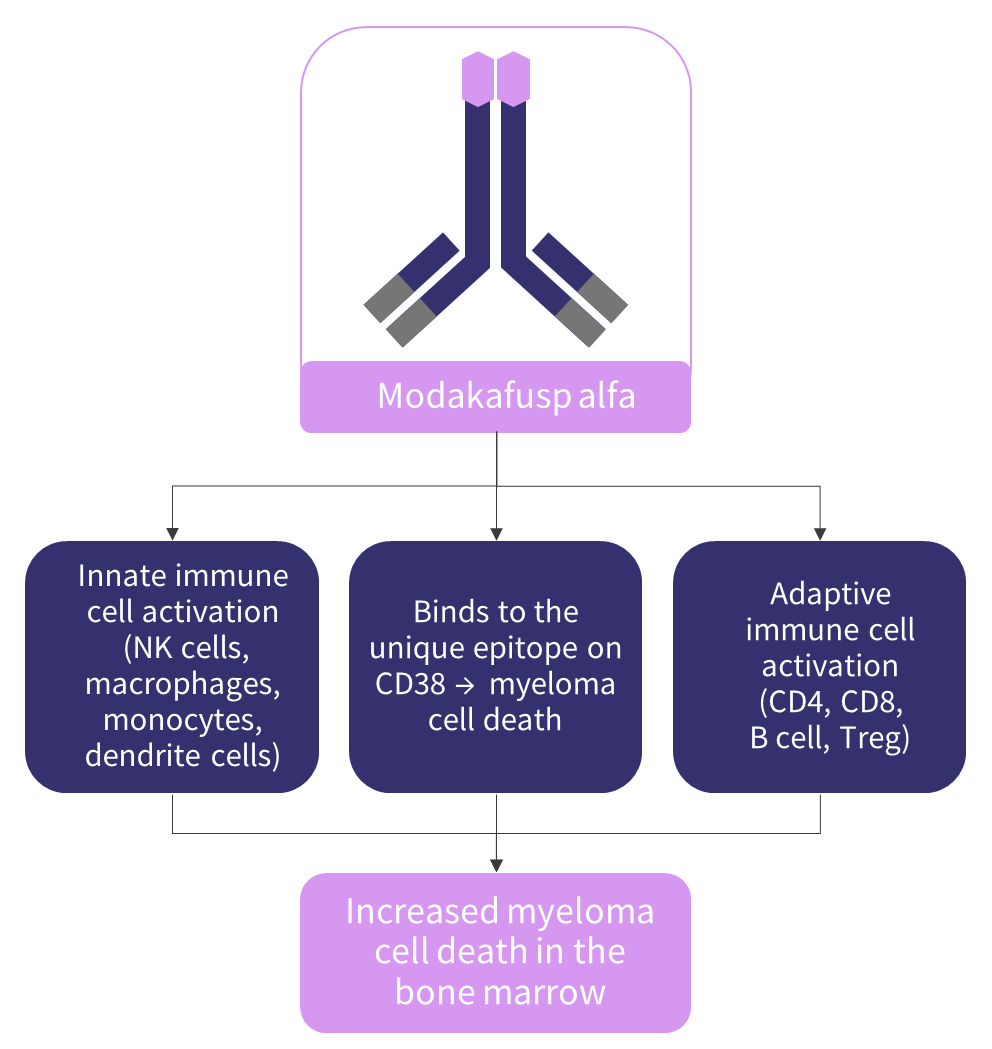
NK, natural killer.
*Adapted from Vogel.2
Study design and endpoints
- Design3: An ongoing first-in-human, phase 1/2, open-label, randomized study of modakafusp alpha as a single agent and in combination with dexamethasone in patients with RRMM in the United States (NCT03215030); the study consists of two parts (Part 1 and Part 2), with an expected enrollment of 51 and 100 patients, respectively
- Eligibility criteria3: Age ≥18 years; disease progression having previously received ≥3 lines of therapy; refractory to or intolerant of at least one proteasome inhibitor and a least one immunomodulatory drug; European Cooperative Oncology Group (ECOG) performance status of 0 to 1
- Endpoints3: Incidence of treatment-emergent adverse events (TEAEs), serious adverse events, dose-limiting toxicities, maximum tolerated dose or optimal biological dose, pharmacokinetics, pharmacodynamics, efficacy
Preliminary results from the ongoing first-in-human, phase I trial of modakafusp alfa in patients with RRMM show clinical activity in response to single-agent therapy with doses starting at 0.1 mg/kg weekly.1
At the 63rd American Society of Hematology (ASH) Annual Meeting and Exposition, Dan Vogl presented updated interim results of the first-in-human outcomes of modakafusp alfa, focusing on outcomes from an expansion cohort with dosing every 4 weeks (Q4W) in patients with RRMM.2 The key findings are summarized below.
Results2
Overall, 29 patients were treated with 1.5 mg/kg Q4W modakafusp alfa: five in the dose-escalation part and 24 in the ongoing dose-expansion part. The analysis includes data from all 29 patients.
Safety
- Grade 3 or higher TEAEs were reported in 18 patients (75%).
- The most frequently reported hematologic TEAEs (>25%) were neutropenia, leukopenia, decreased lymphocyte count, anemia, and thrombocytopenia.
Overall response rate
- Modakafusp alfa has shown promising results in heavily pre-treated patients with RRMM, including some who have received an anti-CD38 mAb in their most recent line of treatment.
- Overall, 38% of patients responded to the treatment, with 28% achieving at least a very good partial response. Further details on best response and by patient cohort are presented in Figure 2.
Figure 2. ORR in patients who received 1.5 mg/kg Q4W modakafusp alfa*
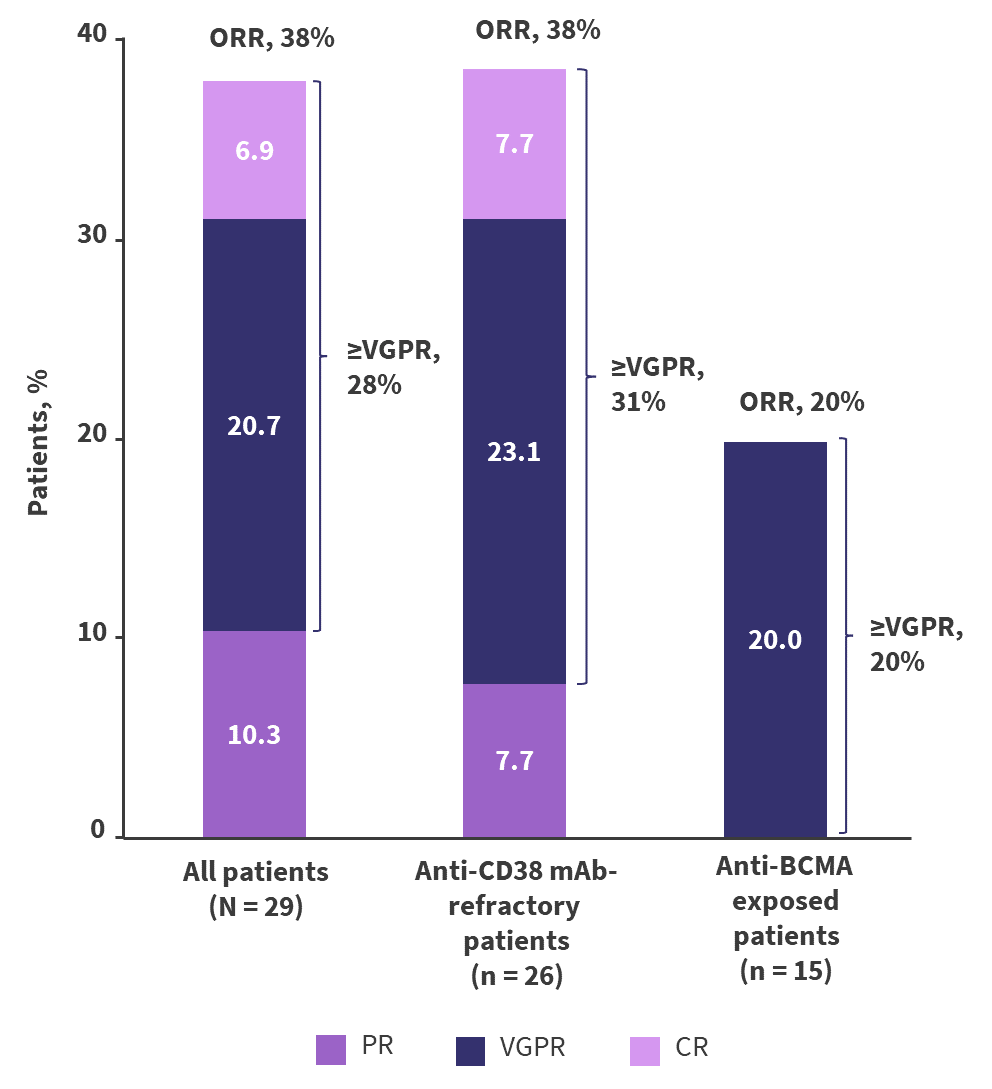
BCMA, B-cell maturation antigen; CR, complete response; ORR, overall response rate; PR, partial response; Q4W, every 4 weeks; VGPR, very good partial response.
*Adapted from Vogel.2
Duration of response
- Among all 29 patients, the median progression-free survival was 5.7 months.
- Among 11 patients with a partial response or better:
- The median time to response was 1 month.
- The median time to best response was 2 months.
- The median duration of response has not been reached.
Conclusion
Modakafusp alfa represents a promising candidate for the treatment of patients with RRMM. The investigators considered that modakafusp alfa had a manageable safety profile, with anti-myeloma activity in heavily pre-treated patients.2 The dose optimization and potential combinations are currently being explored in the dose-escalation part of the study.3
- Vogl DT, Kaufman JL, Holstein SA, et al. Modakafusp alfa (TAK-573), an immunocytokine, shows clinical activity in patients with relapsed/refractory multiple myeloma; updated results from a first-in-human phase 1 study. Abstract #898. Presented at: 63rd American Society of Hematology Annual Meeting and Exposition; Dec 13, 2021; Atlanta, US.
- Vogl DT. Modakafusp alfa (TAK-573), an immunocytokine, shows clinical activity in patients with relapsed/refractory multiple myeloma; updated results from a first-in-human phase 1 study. Oral abstract #898. 63rd American Society of Hematology Annual Meeting and Exposition; Dec 13, 2021; Atlanta, US.
- Clinicaltrials.gov. A study to investigate the safety, tolerability, efficacy, pharmacokinetics, and immunogenicity of TAK-573 in participants with refractory multiple myeloma (MM). https://clinicaltrials.gov/ct2/show/NCT03215030. Updated Oct 22, 2021. Accessed Apr 04, 2022.
Your opinion matters
17 votes - 12 days left ...
Related articles
Newsletter
Subscribe to get the best content related to multiple myeloma delivered to your inbox






